地黄苷元 | 1672-46-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:222 °C(lit.)
-
沸点:435.71°C (rough estimate)
-
密度:1.0639 (rough estimate)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
颜色/状态:Prisms from ethyl acetate
-
蒸汽压力:8.41X10-15 mm Hg at 25 °C (est)
-
稳定性/保质期:
Stable under recommended storage conditions.
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
碰撞截面:195.1 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:28
-
可旋转键数:1
-
环数:5.0
-
sp3杂化的碳原子比例:0.869
-
拓扑面积:87
-
氢给体数:3
-
氢受体数:5
ADMET
安全信息
-
危险等级:6.1(a)
-
危险品标志:T+
-
安全说明:S36/37/39,S45
-
危险类别码:R26/27/28
-
WGK Germany:3
-
危险品运输编号:UN 2811 6.1/PG 1
-
RTECS号:FH5390000
-
包装等级:II
-
危险类别:6.1(a)
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3β-Acetoxy-12β,14-dihydroxy-5β,14β-card-20(22)-enolid 80680-86-0 C25H36O6 432.557 毛地黄毒苷配基 (+)-digitoxigenin 143-62-4 C23H34O4 374.521 4-[(3S,5R,8R,9S,10S,12R,13S,14S,17R)-3-[(2R,4S,5S,6R)-4,5-二羟基-6-甲基四氢吡喃-2-基]氧基-12,14-二羟基-10,13-二甲基-1,2,3,4,5,6,7,8,9,11,12,15,16,17-十四氢环戊烯并[a]菲-17-基]-5H-呋喃-2-酮 Digoxigenin mono-digitoside 5352-63-6 C29H44O8 520.664 地高辛 digoxin 20830-75-5 C41H64O14 780.951 β-乙酰地高辛 beta-Acetyldigoxin 5355-48-6 C43H66O15 822.988 α-乙酰基地高辛 3'''-acetyldigoxin 5511-98-8 C43H66O15 822.988 洋地黄毒苷 digitoxin 71-63-6 C41H64O13 764.951 毛花苷C lanatoside C 17575-22-3 C49H76O20 985.13 2-乙酰氨基-1,2,5-三脱氧-1,5-亚氨基-d-葡萄糖醇 acetyldigitoxin 1111-39-3 C43H66O14 806.989 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (3alpha,5beta,12beta)-3,12,14-三羟基心甾-20(22)-烯内酯 3α-hydroxydigoxigenin 465-14-5 C23H34O5 390.52 (5beta)-12,beta,14-二羟基-3-氧代心甾-20(22)-烯内酯 3-dehydrodigoxigenin 4442-17-5 C23H32O5 388.504 —— 3-Dehydro-digoxigenin 4442-17-5 C23H32O5 388.504 地高辛配基-3,12-二乙酸酯 3,12-Diacetyl-digoxingenin 6078-59-7 C27H38O7 474.595 —— (20R)-3β,12β,14-trihydroxy-5β,14β-cardanolide —— C23H36O5 392.536
反应信息
-
作为反应物:描述:地黄苷元 在 吡啶 、 chromium(VI) oxide 、 氯化亚砜 、 溶剂黄146 、 铂 作用下, 生成 3β,11β-Diacetoxy-12-oxo-5β,17αH-aetiansaeuremethylester参考文献:名称:合成von3β,12β-Diacetoxy-11-keto-ätiansäure-methyl-ester和zweier异构体Ätiansäureester。Gallensäurenund verwandte Stoffe,49岁。摘要:地高辛中的地高辛配基在schlechter Ausbeute ua das 12-O-Acetyldigoxigenin(II)中。3,12-二-O-乙酰基洋地黄毒苷(VI)ur 3-β-Acatoxy-12-酮基-天青-甲基酯(VIII)abgebaut,und daraus wurde durch Bromieren 3,β-乙酰氧基-12-酮基11ξ-溴-ätiansäure-甲酯(IX)hergestellt。DOI:10.1002/hlca.19560390629
-
作为产物:参考文献:名称:洋地黄苷,洋地黄A,B和C摘要:DOI:10.1002/hlca.193301601136
文献信息
-
[EN] COMPOSITIONS AND METHODS COMPRISING SUBSTITUTED 2-AMINOIMIDAZOLES<br/>[FR] COMPOSITIONS ET PROCÉDÉS COMPRENANT DES 2-AMINOIMIDAZOLES SUBSTITUÉS申请人:CURZA GLOBAL LLC公开号:WO2018184019A1公开(公告)日:2018-10-04The present invention presents 2-(acylamino)imidazoles with therapeutic activity, including selective activity against cancer cells, and compositions comprising them. Methods of using and preparing the 2-(acylamino)imidazoles are also presented.
-
[EN] METHODS AND COMPOSITIONS FOR SUBSTANCE USE DISORDER VACCINE FORMULATIONS AND USES THEREOF<br/>[FR] MÉTHODES ET COMPOSITIONS DESTINÉES À DES FORMULATIONS VACCINALES CONTRE DES TROUBLES LIÉS À UNE SUBSTANCE, ET LEURS UTILISATIONS申请人:MOLECULAR EXPRESS INC公开号:WO2018231706A1公开(公告)日:2018-12-20The present invention relates to vaccine compositions for treatment of substance use disorders, methods for the manufacture thereof, and methods for the use thereof to treat an animal. These compositions comprise a hapten conjugated via a linker to a protein scaffold and mixed with a particulate carrier and at least one immunostimulatory adjuvant molecule.本发明涉及用于治疗物质使用障碍的疫苗组合物,其制造方法以及用于治疗动物的方法。这些组合物包括通过连接剂与蛋白质支架结合的半抗原,并与颗粒载体和至少一种免疫刺激性佐剂分子混合。
-
[EN] IMMUNOPHILIN BINDING AGENTS AND USES THEREOF<br/>[FR] AGENTS DE LIAISON À L'IMMUNOPHILINE ET LEURS UTILISATIONS申请人:UNIV CALIFORNIA公开号:WO2020163594A1公开(公告)日:2020-08-13Described herein, inter alia, are immunophilin binding compounds and methods of treating CNS diseases, including co-administering outside the CNS of a subject an anti-CNS disease drug and a compound described herein.本文描述了免疫蛋白结合化合物以及治疗中枢神经系统疾病的方法,包括在主体的中枢神经系统外联合给予一种抗中枢神经系统疾病药物和本文描述的化合物。
-
[EN] HETEROBIFUNCTIONAL ESTERS FOR USE IN LABELING TARGET MOLECULES<br/>[FR] ESTERS HÉTÉROBIFONCTIONNELS À UTILISER DANS MARQUAGE DE MOLÉCULES CIBLES申请人:LIFE TECHNOLOGIES CORP公开号:WO2012134925A1公开(公告)日:2012-10-04The present disclosure is directed to a reactive ester agent for conjugating a click-reactive group to a carrier molecule or solid support. The reactive ester agent has the general formula IA, wherein the variables R1, R2, R3, Ra and L are described throughout the application.本发明涉及一种用于将点击反应性基团共轭到载体分子或固体支持物上的反应性酯试剂。该反应性酯试剂具有一般公式IA,其中变量R1、R2、R3、Ra和L在申请中有所描述。
-
Effects of cyclodextrins on the acid hydrolysis of digoxin作者:K UEKAMA、T FUJINAGA、F HIRAYAMA、M OTAGIRI、Y KURONO、K IKEDADOI:10.1111/j.2042-7158.1982.tb04690.x日期:2011.4.12
Abstract The effects of three cyclodextrins (α-, β-, γ-CyD) on the acid hydrolysis of digoxin were examined. From the high performance liquid chromatographic tracing of each of the four components (digoxin, bisdigitoxoside, monodigitoxoside, digoxigenin) in reaction mixtures, the individual rate constants (k1-k6) were determined by analogue computer simulation. The hydrolysis was suppressed by CyDs in the order of β->γ->α-CyD, where β-CyD inhibited the appearance rates of digoxigenin (k3, k5, and k6) significantly. In the dissolution study of digoxin tablets, the increase in dissolution rate and decrease in acid hydrolysis were attained by inclusion complexation. The data are presented suggesting that CyDs are useful for improving the oral bioavailability of digoxin.
表征谱图
-
氢谱1HNMR
-
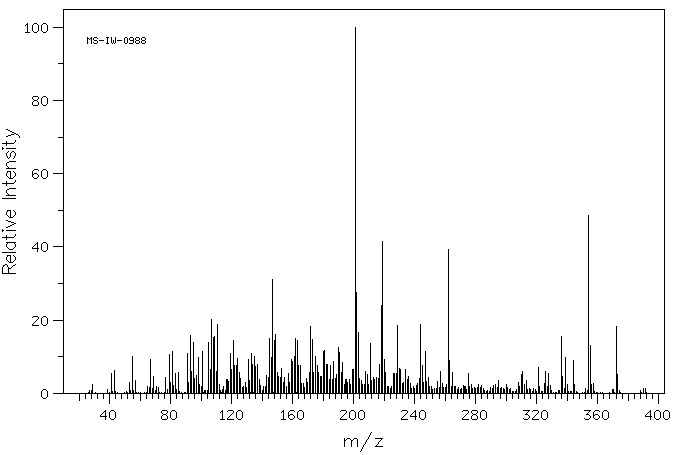
质谱MS
-
碳谱13CNMR
-
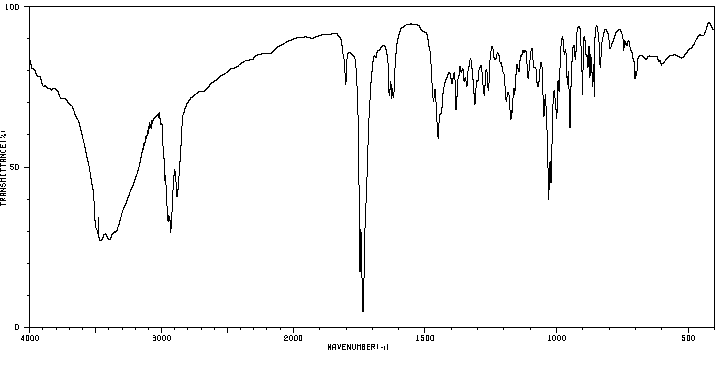
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息