三氯一茂钛 | 1270-98-0
中文名称
三氯一茂钛
中文别名
环戊二烯基三氯化钛(IV);(Η-环戊二烯基)三氯化钛;环戊二烯钛三氯化物;三氯化环戊二烯基钛;(η-环戊二烯基)三氯化钛
英文名称
cyclopentadienyl titanium(IV) trichloride
英文别名
CpTiCl3;cyclopentadienyltitanium trichloride;cyclopenta-1,3-diene;titanium(4+);trichloride
CAS
1270-98-0
化学式
C5H5Cl3Ti
mdl
——
分子量
219.334
InChiKey
QOXHZZQZTIGPEV-UHFFFAOYSA-K
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.47
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:No
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S28,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2931900090
-
RTECS号:XR1927300
-
包装等级:II
-
危险类别:8
-
危险品运输编号:UN 3261 8/PG 2
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H314
-
储存条件:请将贮藏器保持密封状态,并存放在阴凉干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
| Name: | Cyclopentadienyltitanium Trichloride Material Safety Data Sheet |
| Synonym: | Cyclopentadienyltitanium(IV) trichloride; Cyclopentadienyltrichlorotitanium; pi-Cyclopentadienyltrichlorotitanium(IV); Titanium, trichlorocyclopentadienyl-; Titanium, trichloro-pi-cyclopentadienyl-; Titanium, trichloro(eta(sup 5)-2,4-c |
| CAS: | 1270-98-0 |
Synonym:Cyclopentadienyltitanium(IV) trichloride; Cyclopentadienyltrichlorotitanium; pi-Cyclopentadienyltrichlorotitanium(IV); Titanium, trichlorocyclopentadienyl-; Titanium, trichloro-pi-cyclopentadienyl-; Titanium, trichloro(eta(sup 5)-2,4-c
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1270-98-0 | Cyclopentadienyltitanium Trichloride | 100 | unlisted |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.Corrosive.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye burns.
Skin:
Causes skin burns.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause headache. May cause nausea and vomiting.
Inhalation:
May cause irritation of the respiratory tract with burning pain in the nose and throat, coughing, wheezing, shortness of breath and pulmonary edema. Causes chemical burns to the respiratory tract.
Inhalation may be fatal as a result of spasm, inflammation, edema of the larynx and bronchi, chemical pneumonitis and pulmonary edema.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide. Do NOT get water inside containers.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation. Do not get water inside containers.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Minimize dust generation and accumulation. Do not breathe dust, vapor, mist, or gas. Keep container tightly closed. Do not get on skin or in eyes.
Use with adequate ventilation. Do not allow contact with water.
Discard contaminated shoes. Keep from contact with moist air and steam.
Storage:
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1270-98-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: yellow-orange
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Decomposes
Freezing/Melting Point: 170 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: 210 deg C
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C5H5Cl3Ti
Molecular Weight: 219.36
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, moisture, excess heat, exposure to moist air or water.
Incompatibilities with Other Materials:
Strong acids, strong bases, strong oxidizing agents, strong reducing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, titanium/titanium oxides.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1270-98-0: XR1927300 LD50/LC50:
Not available.
Carcinogenicity:
Cyclopentadienyltitanium Trichloride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, N.O.S.*
Hazard Class: 8
UN Number: 1759
Packing Group: III
IMO
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing Group: III
RID/ADR
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 25 Avoid contact with eyes.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1270-98-0: No information available.
Canada
CAS# 1270-98-0 is listed on Canada's DSL List.
CAS# 1270-98-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1270-98-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
超分子主体材料
反应信息
-
作为反应物:描述:三氯一茂钛 在 (CH3)3SiC5H4(CH2CH2N(CH3)2) 作用下, 以 甲苯 为溶剂, 生成 (cyclopentadienyl)((N,N-dimethylaminoethyl)cyclopentadienyl)TiCl2 * HCl参考文献:名称:复杂化学反应中的Der(N,N-二甲基氨基乙基)环戊二烯基-配体摘要:按照常规方法合成了含有N,N-二甲基氨基乙基环戊二烯基配体的第一钛(III,IV)和锆(IV)配合物。基于1 H-NMR和X射线晶体结构信息描述了二甲基氨基官能团的配位行为。DOI:10.1016/0022-328x(94)05092-p
-
作为产物:参考文献:名称:The application of 13C and 1H NMR spectroscopy to the investigation of the dinitrogen fixation process in the system (η-C5H5)2TiCl2-Mg摘要:DOI:10.1016/0022-328x(83)80218-6
-
作为试剂:参考文献:名称:LIPID NANOPARTICLE COMPOSITIONS AND USES THEREOF摘要:Provided is lipid nanoparticles having a diameter above certain value, as well as the preparation and uses of such compositions. Lipid nanoparticles generally have a diameter of at least 160 nm. Such lipid nanoparticles are useful in the delivery of therapeutic agents, such as nucleic acids, in vivo to none-hepatic organs (e.g., lung) for the treatment or prevention of certain diseases or disorders.公开号:WO2024149229A1
文献信息
-
New amido- and imido-titanium complexes作者:Christina T. Vroegop、Jan H. Teuben、Fr� van Bolhuis、Johannes G. M. van der LindenDOI:10.1039/c39830000550日期:——Me3SiN(H)R (R = Et, Pri, But, or Ph) yields the amido complexes cpTi(Cl)2N(H)R, which react further by HCl abstraction to give the corresponding centrosymmetric binuclear imido complexes [cp(Cl)Ti]2(µ-RN)2; substitution of the chloride ligands by organic groups is possible with the imido complexes, as is reversible reduction to stable anionic complexes.
-
Imidotitanium Complexes as Hydroamination Catalysts: Substantially Enhanced Reactivity from an Unexpected Cyclopentadienide/Amide Ligand Exchange作者:Jeffrey S. Johnson、Robert G. BergmanDOI:10.1021/ja005685h日期:2001.3.1reactivity of 6·py was apparent when it was compared to that of Cp2TiMe2 (2) in the alkyne hydroamination reaction (eq 6). Complex 6·py (3 mol %) rapidly catalyzed the addition of 2,6-dimethylaniline to diphenylacetylene (75 °C, t1/2 < 15 min, [alkyne]0 = 0.43 M, [6·py] = 0.01 M), while complex 2, under identical conditions, yielded no enamine product after 12 h. In accord with Doye’s observations,3 hydroamination在碳-碳双键或三键上添加 N-H 键可能是烷基化胺的最直接和最有效的方法之一。已经付出了相当大的努力来寻找能够催化这种理想转化的过渡金属配合物,虽然已经取得了显着的成功,但已证明该问题的一般解决方案难以捉摸。 1 因此,加氢胺化反应的发现和开发仍然很重要目标。相对较少的早期过渡金属络合物已被证明是加氢胺化催化剂前体。其中包括我们小组 2 和 Doye 的 3,4(方程 1)较早研究的双(环戊二烯基)锆和钛炔烃加氢胺化催化剂。在这两个系统中,都假定关键中间体是 16 电子亚胺配合物 Cp2M=NR。本次通讯的目的是报告钛基亚胺配合物 5,6,它们可作为丙二烯和炔烃加氢胺化反应的催化剂前体。 7-9 详细的机械研究揭示了一种意想不到的环戊二烯/酰胺配体交换反应,该反应可转化双环戊二烯基钛前体(Cp2TiLn) 转化为单环戊二烯基钛(酰胺基)络合物(Cp(ArNH)TiLn)10,表现出比 Cp2TiLn
-
Half-titanocene 5-t-butyl-2-(1-(arylimino)methyl)quinolin-8-olate chlorides: Synthesis, characterization and ethylene (co-)polymerization behavior作者:Qing Yan、Wenhong Yang、Langqiu Chen、Lin Wang、Carl Redshaw、Wen-Hua SunDOI:10.1016/j.jorganchem.2013.12.021日期:2014.3A series of half-titanocene chloride complexes bearing 5-t-butyl-2-(1-(arylimino)methyl)quinolin-8-olate ligands (L), CpTiLCl2, has been synthesized in acceptable yields by the stoichiometric reaction of CpTiCl3 with the respective potassium 5-t-butyl-2-(1-(arylimino)methyl)quinolin-8-olate. All half-titanocene complexes were fully characterized by elemental analysis and NMR spectroscopy, and the molecular通过CpTiCl的化学计量反应,以可接受的产率合成了一系列带有5-叔丁基-2-(1-(芳基)甲基)喹啉-8-油酸酯配体(L)CtTiLCl 2的半钛茂氯化物配合物。3与相应的钾5-吨丁基-2-(1-(芳基亚氨基)甲基)喹啉-8-醇钠。通过元素分析和NMR光谱对所有半钛茂金属配合物进行了充分表征,并确定了代表性配合物C1和C2的分子结构通过单晶X射线衍射证实在钛处为伪八面体。当用甲基铝氧烷(MAO)或改性甲基铝氧烷(MMAO)活化时,所有钛配合物均表现出良好的乙烯聚合活性(高达4.8×10 5 g mol - 1(Ti)h -1)。将所得到的聚乙烯显示出超高分子量(高达11.82×10 5 克摩尔- 1)具有窄多分散性。此外,通过在所得聚乙烯中掺入几个百分比的共聚单体,可以实现乙烯与1-己烯或1-辛烯的有效共聚。
-
Monocyclopentadienyl Phenoxido–Amino and Phenoxido–Amido Titanium Complexes: Synthesis, Characterisation, and Reactivity of Asymmetric Metal Centre Derivatives作者:Giuseppe Alesso、Martial Sanz、Marta E. G. Mosquera、Tomás CuencaDOI:10.1002/ejic.200800553日期:2008.10/ethyl ether solution. The reaction of TiCpCl 3 with 2a in the presence of 2.5 equiv. of NEt 3 in a polar solvent (thf, CH 2 Cl 2 or toluene) at room temperature affords TiCp[Ph(H)NCH2(3,5-tBu 2 C 6 H 2 -2-O)]Cl (6a) as a mixture of two stereoisomers. All the reported compounds were characterised by the usual analytical and spectroscopic methods and the molecular structures of 2a, 2d, 2e and 3d were酚亚胺衍生物的还原 R'N=CH(3,5-R 2 C 6 H 2 -2-OH) (R = tBu; R' = C 6 H 5 la, p-MeC 6 H 4 1b, Cy 1c, tBu 1d, 2,6-Me 2 C 6 H 3 1e;R = H;R' = p-MeC 6 H 4 1f;Cy = 环己基)与 MBH 4 (M = Li, Na) 或 AlLiH 4 in乙醚或 thf 在室温下得到苯酚-胺化合物 R'NHCH2(3,5-R2C 6 H 2 -2-OH) 2a-c 和 2e,f。NR-[2,4-二-叔丁基]苯并-1-oxa-3-azine物质(R = tBu 2dl, 2,6-Me 2 C 6 H 3 2el)通过2的曼尼希反应获得, 4-二叔丁基苯酚与 RNH 2 在回流甲醇中。中间体2dl在室温下在乙醇中转化为N-叔丁基[2-羟基-3,5-二-叔丁基]苄胺(2d),而2e
-
Coordination Chemistry of Stable Radicals: Homolysis of a Titanium−Oxygen Bond作者:Kuo-Wei Huang、Robert M. WaymouthDOI:10.1021/ja0264854日期:2002.7.1Thermolysis of Cp2TiCl(TEMPO) (TEMPO = 2,2,6,6-tetramethylpiperidine-1-oxyl) at 60 degrees C in a benzene/CCl4 mixture generates Cp2TiCl2. Kinetic studies implicate a mechanism involving the reversible cleavage of a Ti-O bond to generate the TEMPO radical and Cp2TiCl, which is trapped by CCl4 to give Cp2TiCl2. The rate of this reaction is strongly inhibited by added TEMPO and increases with increasing
表征谱图
-
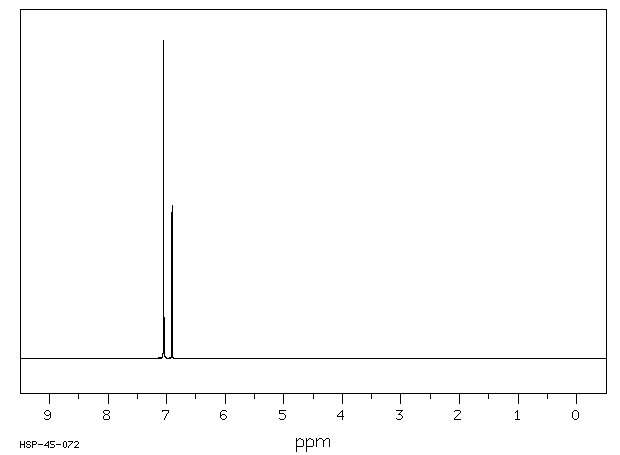
氢谱1HNMR
-
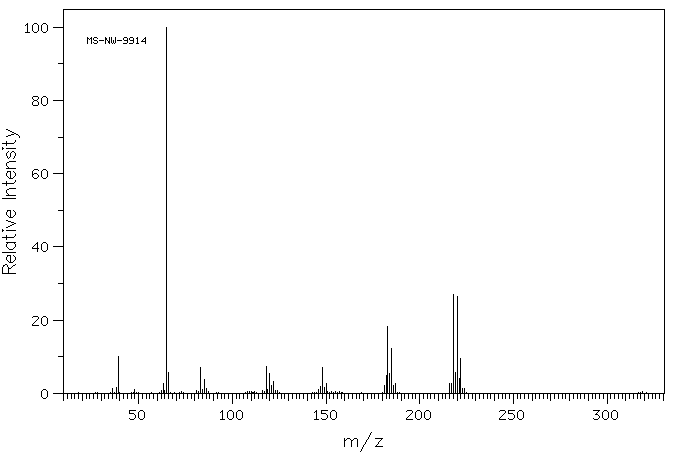
质谱MS
-
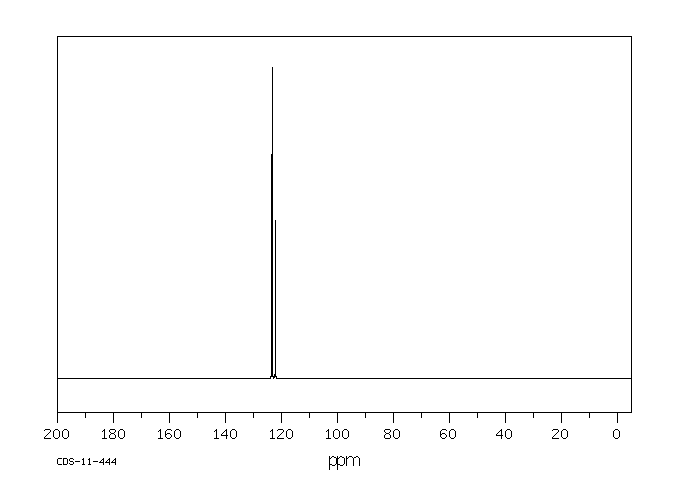
碳谱13CNMR
-
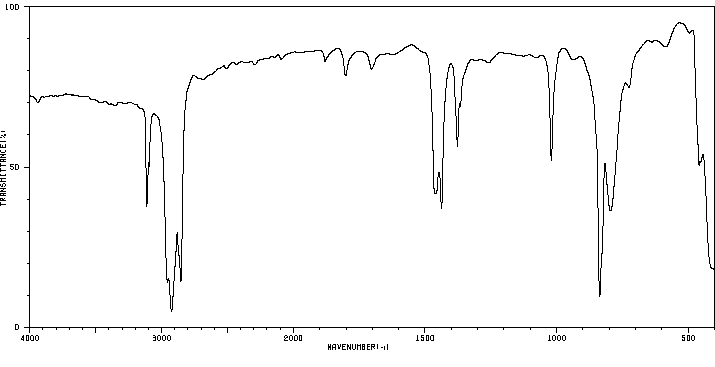
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄原酸环癸酯
高纯三甲基锑
顺式-二氯二(环丙胺)铂(II)
顺式-二氯二(乙二胺)氯化铑(1+)
顺式-二(环己基丁氨合)二氯铂(II)
顺式-二(异丙基氨合)二氯铂(II)
顺式-(2-氨基甲基-1-环戊基氨合)二氯铂(II)
顺二氯二羰基铂(II)
顺-二氯双(乙二胺)氯化铱
雷(酸)汞[含水或水加乙醇≥20]
间碳硼烷-9-硫醇
镍,加合(7:2)钪
镉二(二戊基二硫代氨基甲酸盐)
镁,溴-6-庚烯基-
manganese carbide
butyl manganese bromide
锡烷,氯二环己基-
锡四丁醇
锑,(1:1)混合物和钪
锌叔-丁氧化物
锌,溴-1-丙烯基-,(E)-
锇,加合(2:1)钪
锆酸四丁酯
锂丁酯
锂4-异丙氧基-2-甲基-丁烷-2-醇
锂1-丁醇
锂(三氟甲基)乙炔化物
锂(3-氨基丙基)酰胺
铼五羰基碘化物
铼五羰基
银(I)2-羟基乙烷-1-硫醇盐
铯三氯三羰基锇
铬三乙二胺
铬,五羰基(环己胺)-,(OC-6-22)-
铬,二(乙酰腈)二氯-
铝,加合(3:1)钪
铜-乙二胺络合物
铜(II)乙二胺
铜(I)乙炔化物
铍,环戊-1,3-二烯,溴化
铊N,N-二正丁胺
铊,甲氧基二甲基-
铂(2+)二氯化3-甲基丁烷-1,2-二胺(1:1)
铁(3+)三(1-丁醇)
铁(2+)1,1'-(硫烷二基二-1,1-乙二基)二-2,4-环戊二烯化
铀,三甲基-
钾,[三(三甲基甲硅烷基)甲基]-
钴四异硫氰酸酯
钴,乙烷-1,2-二胺
钠辛基二硫代氨基甲酸酯