1-(4-碘苯基)-1H-吡咯-2,5-二酮 | 65833-01-4
中文名称
1-(4-碘苯基)-1H-吡咯-2,5-二酮
中文别名
——
英文名称
1-(4-iodophenyl)-1H-pyrrole-2,5-dione
英文别名
N-(4-Iodophenyl)maleimide;1-(4-iodophenyl)pyrrole-2,5-dione
CAS
65833-01-4
化学式
C10H6INO2
mdl
MFCD00022575
分子量
299.068
InChiKey
GIXQASIEVHMYQH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2925190090
SDS
1.1 产品标识符
: N-(4-Iodophenyl)maleimide
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
严重的眼损伤 (类别1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H302 吞咽有害。
H318 造成严重眼损伤。
警告申明
预防
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P330 漱口。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C10H6INO2
分子式
: 299.07 g/mol
分子量
组分 浓度或浓度范围
N-(4-Iodophenyl)maleimide
-
CAS 号 65833-01-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 碘化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 2.396
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 引起眼睛烧伤。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: N-(4-Iodophenyl)maleimide
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
严重的眼损伤 (类别1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H302 吞咽有害。
H318 造成严重眼损伤。
警告申明
预防
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P330 漱口。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C10H6INO2
分子式
: 299.07 g/mol
分子量
组分 浓度或浓度范围
N-(4-Iodophenyl)maleimide
-
CAS 号 65833-01-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 碘化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 2.396
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 引起眼睛烧伤。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(4-碘苯基)吡咯烷-2,5-二酮 1-(4-iodophenyl)pyrrolidine-2,5-dione 94590-85-9 C10H8INO2 301.084
反应信息
-
作为反应物:描述:1-(4-碘苯基)-1H-吡咯-2,5-二酮 以 四氢呋喃 为溶剂, 反应 24.0h, 生成 3-(((1-dodecyl-1H-1,2,3-triazol-4-yl)methyl)amino)-1-(4-iodophenyl)pyrrolidine-2,5-dione参考文献:名称:Synthesis and applications of 4-substituted 1-(4-iodophenyl)pyrrolidine-2,5-diones摘要:The synthesis of 4-substituted 1-(4-iodophenyl)pyrrolidine-2,5-dione derivatives was achieved through an addition reaction between amines and a thiol in the presence of PMDTA as a base and a copper salt. The derivatives containing a terminal acetylene moiety were converted to the corresponding 1,4-triazolyl derivatives. The 1-(4-iodophenyl)pyrrolidine-2,5-dione derivatives were functionalized through oxidation alkylation and allylation reactions. In general, the compounds were obtained in moderate-to-good yields. (C) 2015 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2015.05.066
-
作为产物:描述:参考文献:名称:吲哚和吡咯的可见光介导的脱芳香化作用,以制得药品和农药。摘要:借助于可见光和氧气已经实现了吲哚衍生物向相应的靛红衍生物的脱芳香化作用。应该注意的是,对于合成药物和生物活性化合物而言,伊斯汀衍生物非常重要。值得注意的是,这种化学方法与N保护和无保护的吲哚均具有出色的表现。另外,该方法还可以应用于在单个步骤中对吡咯衍生物进行脱芳香化以生成环状酰亚胺。后来,这种方法学被用于合成四种药物和一种名为双硫毒素B的农药。详细的机理研究揭示了氧气和光催化剂的实际作用。DOI:10.1002/chem.201904168
文献信息
-
Reduction of Activated Alkenes by P <sup>III</sup> /P <sup>V</sup> Redox Cycling Catalysis作者:Lars Longwitz、Thomas WernerDOI:10.1002/anie.201912991日期:2020.2.10using a phosphetane oxide catalyst in the presence of a simple organosilane as the terminal reductant and water as the hydrogen source. Quantitative hydrogenation was observed when 1.0 mol % of a methyl‐substituted phosphetane oxide was employed as the catalyst. The procedure is highly selective towards activated double bonds, tolerating a variety of functional groups that are usually prone to reduction
-
Synthesis and in Vitro Evaluation of N-Substituted Maleimide Derivatives as Selective Monoglyceride Lipase Inhibitors作者:Nicolas Matuszak、Giulio G. Muccioli、Geoffray Labar、Didier M. LambertDOI:10.1021/jm900461w日期:2009.12.10action is quickly terminated via enzymatic hydrolysis catalyzed by monoglyceride lipase (MGL). Regulating its endogenous level could offer therapeutic opportunities; however, few selective MGL inhibitors have been described so far. Here, we describe the synthesis of N-substituted maleimides and their pharmacological evaluation on the recombinant human fatty acid amide hydrolase (FAAH) and on the purified内源性大麻素2-花生四烯酸甘油酯(2-AG)在许多生理过程中起着重要作用,其作用通过甘油单酯脂肪酶(MGL)催化的酶促水解迅速终止。调节其内源水平可以提供治疗机会;然而,到目前为止,几乎没有描述选择性的MGL抑制剂。在这里,我们描述了N-取代的马来酰亚胺的合成及其对重组人脂肪酸酰胺水解酶(FAAH)和纯化人MGL的药理评价。先前已经描述了一些N-芳基马来酰亚胺(萨里奥(SM);萨洛(OM);Nevalainen,T .;Poso,A。莱蒂宁(JT);贾文恩(T. Jarvinen)Niemi,R.大鼠小脑膜中2-花生四烯酸甘油水解酶中巯基敏感位点的表征。化学 生物学 2005年,12,649-656),为MGL抑制剂,以及沿着这些线路,我们提出一组新的马来酰亚胺衍生物的那显示出低微摩尔的IC 50和朝向MGL VS FAAH高选择性。然后,研究了结构活性关系,例如,1-biphenyl-4
-
Antifungal, cytotoxic and SAR studies of a series of N-alkyl, N-aryl and N-alkylphenyl-1,4-pyrrolediones and related compounds作者:M. Sortino、F. Garibotto、V. Cechinel Filho、M. Gupta、R. Enriz、S. ZacchinoDOI:10.1016/j.bmc.2011.03.038日期:2011.5The synthesis, in vitro evaluation and SAR studies of 67 maleimides and derivatives acting as antifungal agents are reported. A detailed SAR study supported by theoretical calculations led us to determine that: an intact maleimido ring appears to be necessary for a strong antifungal activity, dissimilarly affected by the substituents in positions 2 and 3. The best activities were shown by 2,3-nonsubstituted报道了67种马来酰亚胺及其衍生物作为抗真菌剂的合成,体外评估和SAR研究。由理论计算支持的详细SAR研究使我们确定:完整的马来酰亚胺环似乎对于强的抗真菌活性是必需的,与位置2和3上的取代基不同。然后是2,3二氯和2-甲基取代的马来酰亚胺。它们都是杀真菌剂,而不是抑真菌剂,增强了其抗真菌活性的重要性。2,3-二甲基和2,3-二苯基-马来酰亚胺具有边际活性或无效活性。N中存在灵活的连接链-苯基烷基马来酰亚胺似乎不是抗真菌活性所必需的,尽管其长度显示出与抗真菌行为的相关性,显示出烷基链为n = 3和n = 4的马来酰亚胺是大多数真菌中最佳的抗真菌活性。苯环上的不同取代基对活性没有明显的影响。化学势性质以及能量的值不能充分区分活性化合物和非活性化合物。尽管如此,发现尽管log P单凭其强度不足以正确预测其抗真菌活性,对同一亚型化合物的抗真菌活性值的比较表明,抗真菌活性随亲脂性的增加而增强。另
-
Methanesulfonic Acid Catalyzed Friedel–Crafts Reaction of Electron-Rich Arenes with N-Arylmaleimides: A Highly Efficient Metal-Free Route To Access 3-Arylsuccinimides作者:Rama Krishna Peddinti、Deepti GairolaDOI:10.1055/s-0040-1706008日期:2021.6Friedel–Crafts reaction is widely used for the C–C bond forming reaction to enable the direct connection of electron-rich arenes to electron-deficient olefins with high regioselectivity. Herein, a highly efficient, metal-free, and environmentally benign F–C strategy of electron-rich arenes with N-arylmaleimides has been developed for the construction of 3-arylsuccinimides in the presence of a green
-
Gold‐Catalyzed 1,2‐Diarylation of Alkenes作者:Chetan C. Chintawar、Amit K. Yadav、Nitin T. PatilDOI:10.1002/anie.202002141日期:2020.7.13Herein, we disclose the gold‐catalyzed 1,2‐diarylation of alkenes through the interplay of ligand‐enabled AuI/AuIII catalysis with the idiosyncratic π‐activation mode of gold complexes. Unlike the classical migratory‐insertion‐based approach to 1,2‐diarylation, the present approach not only circumvents the formation of direct Ar−Ar′ coupling and Heck‐type side products but more intriguingly demonstrates
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
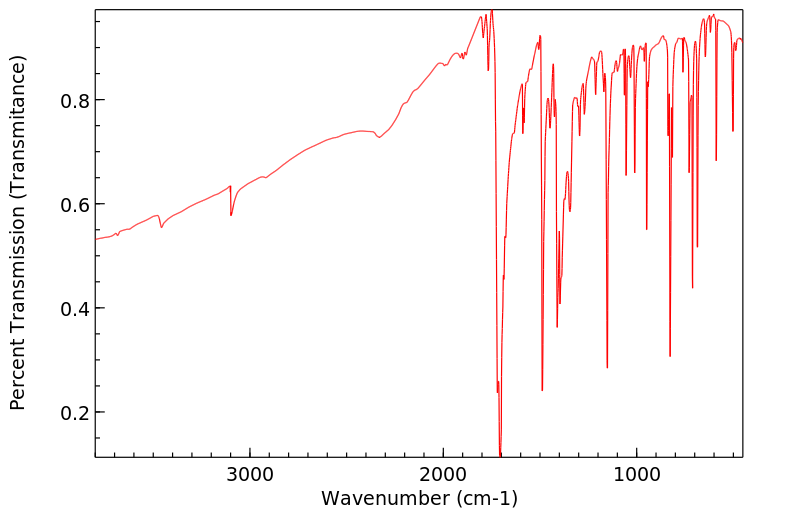
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
颜料红254
颜料橙73
颜料橙 71
赛拉霉素
裂假丝菌素
苯磺酰胺,4-[(2,5-二氢-4-羟基-2-羰基-1,5-二苯基-1H-吡咯-3-基)偶氮]-
苯扎托品氢溴酸盐
苯乙醇,2-(甲氧基甲基)-(9CI)
肼甲硫代酰胺,2-(2,5-二氢-5-羰基-1,2-二苯基-1H-吡咯-3-基)-N-(苯基甲基)-
细交链孢菌酮酸
禾大壮
甲基4-甲酰基-2,3-二氢-1H-吡咯-1-羧酸酯
甲基4-甲氧基-2,5-二氧代-2,5-二氢-1H-吡咯-3-羧酸酯
甲基3-乙烯基-2,5-二氢-1H-吡咯-1-羧酸酯
甲基3,4-二溴-2,5-二氧代-2H-吡咯-1(5H)-羧酸叔丁酯
甲基2-甲基-4,5-二氢-1H-吡咯-3-羧酸酯
甲基2-氮杂双环[3.2.0]庚-3,6-二烯-2-羧酸酯
甲基1-甲基-2,5-二氢-1H-吡咯-3-羧酸酯
甲基(3R)-3-羟基-3,4-二氢-2H-吡咯-5-羧酸酯
烯丙基2,3-二氢-1H-吡咯-1-羧酸酯
氯化烯丙基(3-氯-2-羟基丙基)二甲基铵
氨基甲酰基-2,2,5,5-四甲基-3-吡咯啉-1-氧基
氟酰亚胺
异丙基3,4-二氢-2H-吡咯-5-羧酸酯
己二酸,聚合1,3-二异氰酸基甲基苯,1,2-乙二醇,甲基噁丙环并,噁丙环和1,2-丙二醇
四琥珀酰亚胺金(3+)钾盐
四丁基铵琥珀酰亚胺
吡啶氧杂胺
吡啶,2-[4-(4-氟苯基)-3,4-二氢-2H-吡咯-5-基]-
吡咯烷-2,4-二酮
吡咯布洛芬
叔丁基4-溴-2-氧代-2,5-二氢-1H-吡咯-1-甲酸叔丁酯
叔丁基1H,2H,3H,4H,5H,6H-吡咯并[3,4-C]吡咯-2-甲酸酯盐酸盐
叔-丁基4-(4-氯苯基)-2-氧亚基-2,5-二氢-1H-吡咯-1-甲酸基酯
利收
假白榄内酰胺
二氯马来酸的N-(间甲基苯基)酰亚胺
二-硫代-二(N-苯基马来酰亚胺)
乙基4-羟基-1-[(4-甲氧苯基)甲基]-5-羰基-2-(3-吡啶基)-2H-吡咯-3-羧酸酯
乙基4,5-二氢-1H-吡咯-3-羧酸酯
乙基2-氧代-3,4-二氢-2H-吡咯-5-羧酸酯
乙基2-乙氧基-2-羟基-5-氧代-2,5-二氢-1H-吡咯-1-羧酸酯
乙基2,5-二氢-1H-吡咯-3-羧酸酯
乙基1-苄基-4-羟基-5-氧代-2,5-二氢-1H-吡咯-3-羧酸酯
β.-核-六吡喃糖,1,6-脱水-2-O-(2-氰基苯基)甲基-3-脱氧-4-O-甲基-
[4-(2,5-二氧代吡咯-1-基)苯基]乙酸酯
[3-乙酰基-2-(4-氟-苯基)-4-羟基-5-氧代-2,5-二氢-吡咯-1-基]-乙酸
[3-(甲氧羰基)-2,2,5,5-四甲基-2,5-二氢-1H-吡咯-1-基]氧氮自由基
[3,4-二(溴甲基)-2,2,5,5-四甲基-2,5-二氢-1H-吡咯-1-基]氧氮自由基
[(2R)-1-乙酰基-2,5-二氢-1H-吡咯-2-基]乙腈