高瓜氨酸 | 1190-49-4
物质功能分类
中文名称
高瓜氨酸
中文别名
L-高瓜氨酸;DL-高瓜氨酸
英文名称
N-6-(aminocarbonyl)-L-lysine
英文别名
L-homocitrulline;Homocitrulline;N6-carbamoyl-L-lysine;N6-Carbamoyl-L-lysin;Nε-Carbamoyl-L-lysin;L-2-Amino-6-ureido-capronsaeure;(2S)-2-azaniumyl-6-(carbamoylamino)hexanoate
CAS
1190-49-4
化学式
C7H15N3O3
mdl
MFCD00038143
分子量
189.214
InChiKey
XIGSAGMEBXLVJJ-YFKPBYRVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:211-212℃ (decomposition)
-
沸点:393.0±42.0 °C(Predicted)
-
密度:1.24
-
溶解度:可溶于酸水溶液(少量)、水(少量)
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):-3.9
-
重原子数:13
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:118
-
氢给体数:4
-
氢受体数:4
安全信息
-
安全说明:S26,S36,S36/37/39
-
WGK Germany:3
-
海关编码:2924199090
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:温度范围:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: L-Homocitrulline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: L-Homocitrulline
CAS number: 1190-49-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H15N3O3
Molecular weight: 189.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: L-Homocitrulline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: L-Homocitrulline
CAS number: 1190-49-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H15N3O3
Molecular weight: 189.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 L-哌啶-2-甲酸 pipecolinic acid 3105-95-1 C6H11NO2 129.159
反应信息
-
作为反应物:描述:参考文献:名称:Photocatalytic one-step syntheses of cyclic imino acids by aqueous semiconductor suspensions摘要:DOI:10.1021/jo00308a005
-
作为产物:描述:参考文献:名称:Stevens; Ellman, Journal of Biological Chemistry, 1950, vol. 182, p. 75,76摘要:DOI:
文献信息
-
[EN] LEVORPHANOL PRODRUGS AND PROCESSES FOR MAKING AND USING THEM<br/>[FR] PROMÉDICAMENTS DE LEVORANOL ET LEURS PROCÉDÉS DE FABRICATION ET D'UTILISATION申请人:KEMPHARM INC公开号:WO2018191472A1公开(公告)日:2018-10-18The presently described technology provides compositions of one or more of oxoacids, polyethylene glycols, and vitamin compounds chemically conjugated to levorphanol ((-)-17-methylmorphinan-3-ol) to form novel prodrugs and compositions of levorphanol.
-
Method and compositions for identifying anti-HIV therapeutic compounds申请人:GILEAD SCIENCES, INC.公开号:US20040121316A1公开(公告)日:2004-06-24Methods are provided for identifying anti-HIV therapeutic compounds substituted with carboxyl ester or phosphonate ester groups. Libraries of such compounds are screened optionally using the novel enzyme GS-7340 Ester Hydrolase. Compositions and methods relating to GS-7340 Ester Hydrolase also are provided.
-
PRODRUGS OF OPIOIDS AND USES THEREOF申请人:Franklin Richard公开号:US20110190267A1公开(公告)日:2011-08-04The present invention concerns prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing more consistent pain relief by increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are provided. The invention also provides for decreasing the adverse GI side effects of opioid analgesics.
-
[EN] NOVEL GLUTAMINE ANTAGONISTS AND USES THEREOF<br/>[FR] NOUVEAUX ANTAGONISTES DE LA GLUTAMINE ET LEURS UTILISATIONS申请人:UNIV JOHNS HOPKINS公开号:WO2019071110A1公开(公告)日:2019-04-11Glutamine antagonists and their use for treating oncological, immunological, and neurological diseases are disclosed. Also disclosed are methods for treating an oncological, immunological, infectious or neurological disease or disorder, the method comprising administering to a subject in need of treatment thereof a therapeutically effective amount of a glutamine antagonist of the disclosure or the pharmaceutical composition thereof. Also disclosed are methods of enhancing the effects of an immune checkpoint inhibitor, enabling a subject to respond to an immune checkpoint inhibitor, or enabling the toxicity or the dose or number of treatments with an immune checkpoint inhibitor to be reduced, comprising administering to a subject in need of treatment thereof a therapeutically effective amount of a glutamine antagonist of the disclosure or the pharmaceutical composition thereof, and an immune checkpoint inhibitor. Also disclosed are methods for treating an oncological, immunological, infectious or neurological disease or disorder that is refractory to checkpoint inhibitor therapy, the method comprising administering to a subject in need thereof, and having the refractory disease or disorder, a therapeutically effective amount of a glutamine antagonist of the disclosure or the pharmaceutical composition thereof.
-
Protein kinase inhibitors comprising ATP mimetics conjugated to peptides or pertidomimetics申请人:Livnah Nurit公开号:US20050026840A1公开(公告)日:2005-02-03The present invention provides small molecules having high affinity to the ATP binding site of protein kinases, which are conjugated to apeptide or peptidomimetic moiety which mimics the substrate of PKB. The chimeric compounds according to the present invention preferably serve as PKB inhibitors with improved activity and selectivity. Novel ATP mimetic compounds, particularly isoquinoline derivatives, conjugated with peptides or peptidomimetics are useful as inhibitors of protein kinases for experimental, medical, and drug design purposes. Furthermore, pharmaceutical compositions comprising these protein kinase inhibitors, and methods of using such compositions for treatment and diagnosis of cancers, diabetes, cardiovascular pathologies, hemorrhagic shock, obesity, inflammatory diseases, diseases of the central nervous system, and autoimmune disease, are disclosed.
表征谱图
-
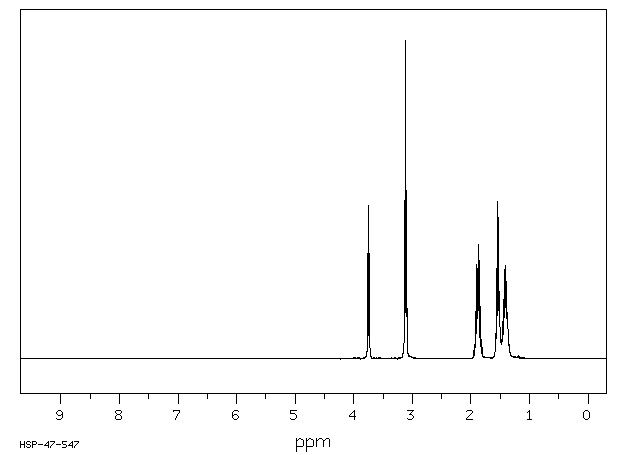
氢谱1HNMR
-
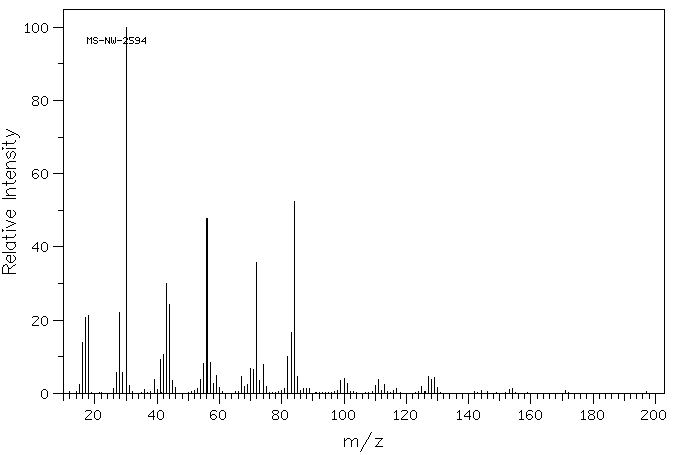
质谱MS
-
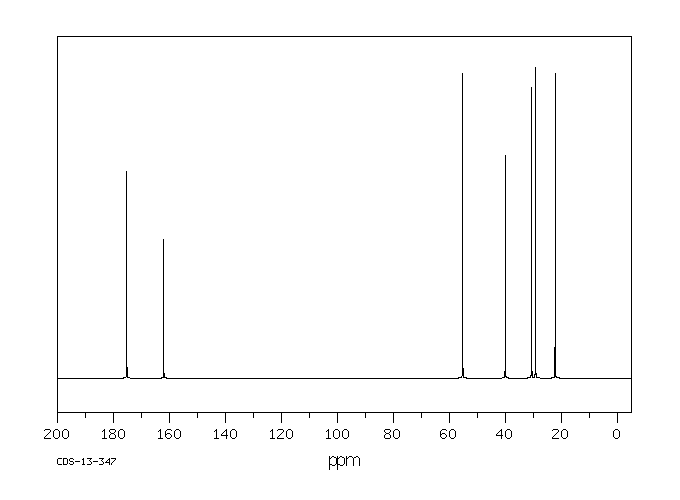
碳谱13CNMR
-
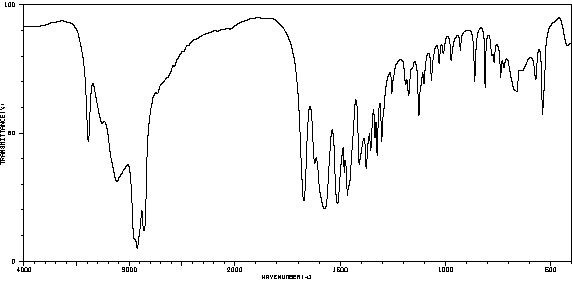
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸