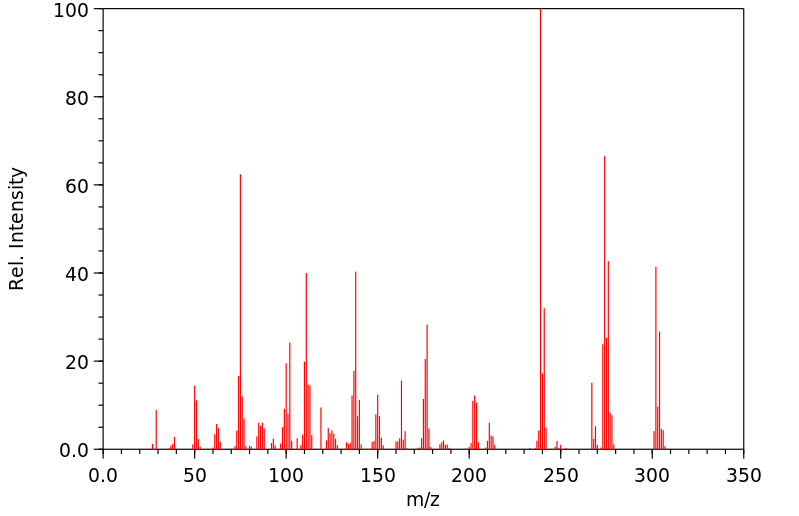
代谢
Lorazepam is hepatically metabolized by CYP450 isoenzymes and extensively conjugated to the 3-0-phenolic glucuronide. This is an inactive metabolite and is eliminated mainly by the kidneys.
来源:DrugBank