十八烷酸 | 506-17-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:14-15 °C(lit.)
-
沸点:150 °C0.03 mm Hg(lit.)
-
密度:0.887 g/mL at 25 °C(lit.)
-
闪点:230 °C
-
溶解度:氯仿(微溶)、乙醇(可溶)、甲醇(微溶)
-
物理描述:Liquid
-
保留指数:2141.4;2116.6;2161.8
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):6.5
-
重原子数:20
-
可旋转键数:15
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2916190090
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:-20°C密封保存,放置于通风、干燥的环境中。
SDS
Section 1. Chemical Product and Company Identification
cis-Vaccenic Acid, synthetic
Common Name/
Trade Name
cis-Vaccenic Acid, synthetic
Section 4. First Aid Measures
Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least
Eye Contact
15 minutes. Get medical attention if irritation occurs.
Wash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops.
Skin Contact
Serious Skin Contact Not available.
Inhalation If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get
medical attention.
Not available.
Serious Inhalation
Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an
Ingestion
unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight
clothing such as a collar, tie, belt or waistband.
Not available.
Serious Ingestion
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
CLOSED CUP: 230°C (446°F).
Flash Points
Not available.
Flammable Limits
These products are carbon oxides (CO, CO2).
Products of Combustion
Fire Hazards in Presence of Slightly flammable to flammable in presence of open flames and sparks, of heat.
Various Substances
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards in
Risks of explosion of the product in presence of static discharge: Not available.
Presence of Various
Substances
SMALL FIRE: Use DRY chemical powder.
Fire Fighting Media
LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
and Instructions
Not available.
Special Remarks on
Fire Hazards
Not available.
Special Remarks on
Explosion Hazards
Section 6. Accidental Release Measures
Absorb with an inert material and put the spilled material in an appropriate waste disposal.
Small Spill
Absorb with an inert material and put the spilled material in an appropriate waste disposal. Finish cleaning by
Large Spill
spreading water on the contaminated surface and allow to evacuate through the sanitary system.
cis-Vaccenic Acid, synthetic
Section 7. Handling and Storage
Keep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not
Precautions
breathe gas/fumes/ vapor/spray. Keep away from incompatibles such as oxidizing agents, alkalis.
Keep container tightly closed. Keep container in a cool, well-ventilated area. Do not store above 0°C (32°F).
Storage
Freeze. Sensitive to light. Store in light-resistant containers.
Section 8. Exposure Controls/Personal Protection
Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
Engineering Controls
respective threshold limit value. Ensure that eyewash stations and safety showers are proximal to the work-station
location.
Personal Protection Safety glasses. Lab coat. Gloves (impervious).
Personal Protection in Case Splash goggles. Full suit. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a specialist
BEFORE handling this product.
of a Large Spill
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Liquid. Not available.
Physical state and O dor
appearance
Not available.
Taste
282.47 g/mole
Molecular Weight
Not available.
Color
Not available.
pH (1% soln/water)
158°C (316.4°F) - 163 C. at 0.4 mm Hg
Boiling Point
14°C (57.2°F) -15 C.
Melting Point
Not available.
Critical Temperature
0.88 - 0.89 (Water = 1)
Specific Gravity
Not available.
Vapor Pressure
Not available.
Vapor Density
Not available.
Volatility
Not available.
Odor Threshold
Not available.
Water/Oil Dist. Coeff.
Not available.
Ionicity (in Water)
Not available.
Dispersion Properties
Not available.
Solubility
Section 10. Stability and Reactivity Data
The product is stable.
Stability
Not available.
Instability Temperature
Conditions of Instability Excess heat, incompatible materials
Incompatibility with various Reactive with oxidizing agents, alkalis.
substances
Not available.
Corrosivity
cis-Vaccenic Acid, synthetic
Sensitive to light.
Special Remarks on
Reactivity
Not available.
Special Remarks on
Corrosivity
Will not occur.
Polymerization
Section 11. Toxicological Information
Absorbed through skin. Eye contact. Ingestion.
Routes of Entry
LD50: Not available.
Toxicity to Animals
LC50: Not available.
Chronic Effects on Humans Not available.
Other Toxic Effects on Slightly hazardous in case of skin contact (irritant), of ingestion, of inhalation.
Humans
Not available.
Special Remarks on
Toxicity to Animals
Not available.
Special Remarks on
Chronic Effects on Humans
Special Remarks on other Acute Potential Health Effects:
Toxic Effects on Humans Skin: Can cause mild to moderate skin irritation.
Eyes: Can cause mild eye irritation.
Inhalation: Inhalation of mist or vapor may cause respiratory tract irritation.
Ingestion: Expected to be a low hazard.
Section 12. Ecological Information
Not available.
Ecotoxicity
Not available.
BOD5 and COD
Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may
arise.
The product itself and its products of degradation are not toxic.
Toxicity of the Products
of Biodegradation
Not available.
Special Remarks on the
Products of Biodegradation
Section 13. Disposal Considerations
Waste must be disposed of in accordance with federal, state and local environmental control
Waste Disposal
regulations.
Section 14. Transport Information
Not a DOT controlled material (United States).
DO T Cl assi fi cati on
Not applicable.
Identification
Not applicable.
Special Provisions for
Transport
cis-Vaccenic Acid, synthetic
DO T (Pi ctograms)
Section 15. Other Regulatory Information and Pictograms
No products were found.
Federal and State
Regulations
California prop. 65: This product contains the following ingredients for which the State of California has found to
California
cause cancer which would require a warning under the statute: No products were found.
Proposition 65
Warnings
California prop. 65: This product contains the following ingredients for which the State of California has found to
cause birth defects which would require a warning under the statute: No products were found.
Other Regulations EINECS: This product is not on the European Inventory of Existing Commercial Chemical Substances.
Canada: Not listed on Canadian Domestic Substance List (DSL) or Canadian Non- Domestic Substance List (NDSL).
China: Not listed on National Inventory.
Japan: Listed on National Inventory (ENCS).
Korea: Not listed on National Inventory (KECI).
Philippines: Not listed on National Inventory (PICCS).
Australia: Not listed on AICS.
WHMIS (Canada) Not controlled under WHMIS (Canada).
Other Classifications
This product is not classified according Not applicable.
DSCL (EEC)
to the EU regulations.
Health Hazard
HMIS (U.S.A.) 1 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
1 0 Reactivity
Health
Reactivity
1
Specific hazard
Personal Protection
B
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
TDG(Canada)
(Pictograms)
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves (impervious).
cis-Vaccenic Acid, synthetic
Lab coat.
Not applicable.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
暂无相关信息
用途暂无相关信息
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 棕榈油酸 Palmitoleic acid 373-49-9 C16H30O2 254.413 (Z,Z)-9,12-十八烷二烯酸二聚物 linoleic acid 60-33-3 C18H32O2 280.451 —— ethyl (11Z)-octadec-11-enoate 137202-20-1 C20H38O2 310.521 11-溴十一酸 11-bromoundecane acid 2834-05-1 C11H21BrO2 265.191 18-溴十八烷酸 18-bromooctadecanoic acid 2536-38-1 C18H35BrO2 363.379 11-十二碳炔酸 dodec-11-ynoic acid 16900-60-0 C12H20O2 196.29 —— 11-octadecynoic acid 19220-40-7 C18H32O2 280.451 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 11-反-十八烯酸 trans-Vaccenic acid 693-72-1 C18H34O2 282.467 顺式-11-十八烯酸甲酯 methyl (Z)-vaccenate 1937-63-9 C19H36O2 296.494 十一烷二酸 undecanedioic acid 1852-04-6 C11H20O4 216.277 —— 11-oxoundecanoic acid 53163-99-8 C11H20O3 200.278 —— 10-Hydroxy-cis-vaccenic acid 143288-76-0 C18H34O3 298.466 —— (Z)-13-hydroxyoctadec-11-enoic acid 521960-22-5 C18H34O3 298.466 —— E-11-hydroxy-octadeca-12-enoic acid 308847-94-1 C18H34O3 298.466 —— 10-hydroxy-trans-11-octadecenoic acid —— C18H34O3 298.466 —— 12-hydroperoxy-(10E)-octadecenoic acid 1158955-43-1 C18H34O4 314.466
反应信息
-
作为反应物:描述:参考文献:名称:Ahmad; Bumpus; Strong, Journal of the American Chemical Society, 1948, vol. 70, p. 3393摘要:DOI:
-
作为产物:参考文献:名称:Ranganathan, S.; Maniktala, Vibha; Kumar, Raaj, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1984, vol. 23, # 12, p. 1197 - 1207摘要:DOI:
文献信息
-
[EN] SYNTHETIC OLIGOGLUCOSAMINES FOR IMPROVEMENT OF PLANT GROWTH AND YIELD<br/>[FR] OLIGOGLUCOSAMINES SYNTHÉTIQUES POUR L'AMÉLIORATION DE LA CROISSANCE ET DU RENDEMENT DE VÉGÉTAUX申请人:DU PONT公开号:WO2015130893A1公开(公告)日:2015-09-03The disclosure provides formulations comprising synthetic oligoglucosamines and methods for improving plant growth and crop yield therewith. These formulations may be applied to propagating materials, including seeds and other regenerable plant parts, including cuttings, bulbs, rhizomes and tubers. They may also be applied to foliage, or soil either prior to or following planting of propagating materials. Such applications may be made alone or in combination with fungicides, insecticides, nematicides and other agricultural agents used to improve plant growth and crop yield.
-
Acyl-group specificity of AHL synthases involved in quorum-sensing in <i>Roseobacter</i> group bacteria作者:Lisa Ziesche、Jan Rinkel、Jeroen S Dickschat、Stefan SchulzDOI:10.3762/bjoc.14.112日期:——
N -Acylhomoserine lactones (AHLs) are important bacterial messengers, mediating different bacterial traits by quorum sensing in a cell-density dependent manner. AHLs are also produced by many bacteria of the marineRoseobacter group, which constitutes a large group within the marine microbiome. Often, specific mixtures of AHLs differing in chain length and oxidation status are produced by bacteria, but how the biosynthetic enzymes, LuxI homologs, are selecting the correct acyl precursors is largely unknown. We have analyzed the AHL production inDinoroseobacter shibae and threePhaeobacter inhibens strains, revealing strain-specific mixtures. Although large differences were present between the species, the fatty acid profiles, the pool for the acyl precursors for AHL biosynthesis, were very similar. To test the acyl-chain selectivity, the three enzymes LuxI1 and LuxI2 fromD. shibae DFL-12 as well as PgaI2 fromP. inhibens DSM 17395 were heterologously expressed inE. coli and the enzymes isolated for in vitro incubation experiments. The enzymes readily accepted shortened acyl coenzyme A analogs,N -pantothenoylcysteamine thioesters of fatty acids (PCEs). Fifteen PCEs were synthesized, varying in chain length from C4 to C20, the degree of unsaturation and also including unusual acid esters, e.g., 2E ,11Z -C18:2-PCE. The latter served as a precursor of the major AHL ofD. shibae DFL-12 LuxI1, 2E ,11Z -C18:2-homoserine lactone (HSL). Incubation experiments revealed that PgaI2 accepts all substrates except C4 and C20-PCE. Competition experiments demonstrated a preference of this enzyme for C10 and C12 PCEs. In contrast, the LuxI enzymes ofD. shibae are more selective. While 2E ,11Z -C18:2-PCE is preferentially accepted by LuxI1, all other PCEs were not, except for the shorter, saturated C10–C14-PCEs. The AHL synthase LuxI2 accepted only C14 PCE and 3-hydroxydecanoyl-PCE. In summary, chain-length selectivity in AHLs can vary between different AHL enzymes. Both, a broad substrate acceptance and tuned specificity occur in the investigated enzymes.N-酰基脱氨核糖乳酸(AHLs)是重要的细菌信使,在细胞密度依赖的方式中介导不同的细菌特征。AHLs也被许多海洋玫瑰细菌组的细菌产生,这在海洋微生物组中占据着一个大的群体。通常,细菌会产生具有不同链长和氧化状态的AHLs的特定混合物,但是生物合成酶LuxI同源物如何选择正确的酰前体大部分是未知的。我们分析了Dinoroseobacter shibae和三株Phaeobacter inhibens菌株中的AHL产生,揭示了菌株特异性混合物。尽管这些物种之间存在很大差异,但脂肪酸谱,即AHL生物合成的酰前体池,非常相似。为了测试酰链选择性,从D. shibae DFL-12的三种酶LuxI1和LuxI2以及从P. inhibens DSM 17395的PgaI2在大肠杆菌中异源表达,并将酶分离用于体外孵育实验。这些酶容易接受缩短的酰辅酶A类似物,即脂肪酸的泛酸半胱氨基乙酰硫酯(PCEs)。合成了15种PCEs,链长从C4到C20不等,不饱和度不同,还包括不寻常的酸酯,例如2E,11Z-C18:2-PCE。后者作为D. shibae DFL-12 LuxI1的主要AHL的前体,2E,11Z-C18:2-脱氨核糖乳酸(HSL)。孵育实验表明,PgaI2接受除C4和C20-PCE之外的所有底物。竞争实验表明,这种酶对C10和C12 PCE有偏好。相比之下,D. shibae的LuxI酶更具选择性。虽然2E,11Z-C18:2-PCE优先被LuxI1接受,但除了较短的饱和C10-C14-PCE之外,所有其他PCE都不被接受。AHL合酶LuxI2仅接受C14 PCE和3-羟基癸酰-PCE。总之,AHL中的链长选择性在不同的AHL酶之间可能有所不同。在研究的酶中既有广泛的底物接受性,也有调节的特异性。 -
METHOD FOR THE SYNTHESIS OF DIACIDS OR DIESTERS FROM NATURAL FATTY ACIDS AND/OR ESTERS申请人:Dubois Jean-Luc公开号:US20100305354A1公开(公告)日:2010-12-02The invention relates to a process for the synthesis of diacids or diesters of general formula ROOC—(CH 2 ) x —COOR, in which n is an integer between 5 and 14, R is either H or an alkyl radical of 1 to 4 carbon atoms, from natural long-chain monounsaturated fatty acids or esters including at least 10 adjacent carbon atoms per molecule of the general formula CH 3 —(CH 2 ) n —CHR 1 —CH 2 —CH═CH—(CH 2 ) p —COOR, in which R is H or an alkyl radical with 1 to 4 carbon atoms, R 1 is either H or OH, and n and p, which are equal or different and are indices between 2 and 11. The method comprises: during a first step, converting the natural fatty acid or ester by pyrolysis or by ethenolysis, into a ω-monounsaturated fatty acid or ester of the general formula CH 2 ═CH—(CH 2 ) m —COOR, in which m is equal to p or p+1, depending on the nature of the processed fatty acid/ester and the conversion used; during a second step, submitting the product thus obtained to a metathesis or homometathesis reaction for obtaining a compound of the general formula ROOC—(CH 2 ) m —CH═CH—(CH 2 ) m —COOR, or cross-metathesis with a compound of formula R 2 OOC—(CH 2 ) r —CH═CH—R 3 , in which R 2 is either H or an alkyl radical front with 1 to 4 carbon atoms, r is either 0 or 1 or 2 and R 3 is H, CH 3 or COOR 2 , thus defining a cyclic or molecule or not, in order to obtain an unsaturated compound of the general formula ROOC—(CH 2 ) m —CH═CH—(CH 2 ) r —COOR 2 , and during a third step, converting the unsaturated compound into a saturated compound by hydrogenation of the double bond.这项发明涉及一种合成一般公式为ROOC—(CH2)x—COOR的二元酸或二元酯的过程,其中n是5到14之间的整数,R是H或1到4个碳原子的烷基自由基,从天然长链单不饱和脂肪酸或酯中合成,这些脂肪酸或酯每分子至少包括10个相邻的碳原子,其一般公式为CH3—( )n—CHR1— —CH═CH—( )p—COOR,其中R是H或1到4个碳原子的烷基自由基,R1是H或OH,n和p相等或不同,是2到11之间的指数。该方法包括:在第一步中,通过热解或乙炔醇解将天然脂肪酸或酯转化为ω-单不饱和脂肪酸或酯,其一般公式为 ═CH—( )m—COOR,其中m等于p或p+1,具体取决于处理的脂肪酸/酯的性质和所使用的转化;在第二步中,将所得产物进行交叉代谢或同交叉代谢反应,以获得一般公式为ROOC—( )m—CH═CH—( )m—COOR的化合物,或与公式为R2OOC—( )r—CH═CH—R3的化合物进行交叉代谢,其中R2是H或1到4个碳原子的烷基自由基,r是0或1或2,R3是H、 或COOR2,从而定义一个循环或不循环的分子,以获得一般公式为ROOC—( )m—CH═CH—( )r—COOR2的不饱和化合物;在第三步中,通过加氢双键将不饱和化合物转化为饱和化合物。
-
METHOD FOR THE SYNTHESIS OF AN OMEGA-AMINO ACID OR ESTER STARTING FROM A MONOUNSATURATED FATTY ACID OR ESTER申请人:Dubois Jean-Luc公开号:US20110224454A1公开(公告)日:2011-09-15The invention relates to a method for the synthesis of ω-amino alkanoic acids or esters thereof starting from unsaturated natural fatty acids passing through an ω-unsaturated nitrile intermediate compound.
-
Amino acid preparation method comprising a step of hydroformylation of an unsaturated fatty nitrile申请人:ARKEMA FRANCE公开号:US10125221B2公开(公告)日:2018-11-13A process for synthesizing an ω-amino acid compound of formula HOOC—(CH2)r+2—CH2NH2, wherein 4≤r≤13 from a monounsaturated fatty nitrile compound of formula CH2═CH—(CH2)r—CN the process comprising: 1) a step of hydroformylation of the mono unsaturated fatty nitrile compound by reacting said nitrile with carbon monoxide and di hydrogen 5e-a5 to obtain a nitrile aldehyde compound of formula HOC—(CH2)r+2-CN, then 2) a step of oxidation, in the presence of dioxygen, of the nitrile aldehyde compound to obtain a corresponding nitrile acid compound of formula HOOC—(CH2)r+2-CN, and 3) a step of reduction of the nitrile acid compound to give an w-amino acid of formula HOOC—(CH2)r+2—CH2NH2.
表征谱图
-
氢谱1HNMR
-
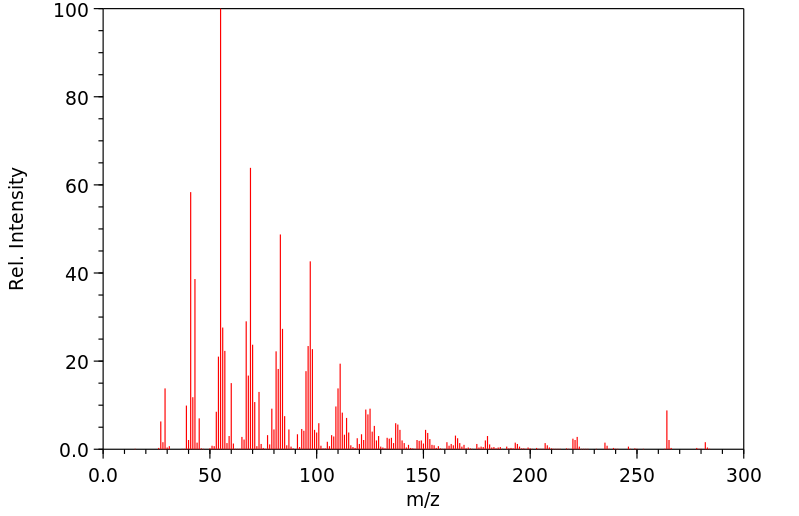
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息