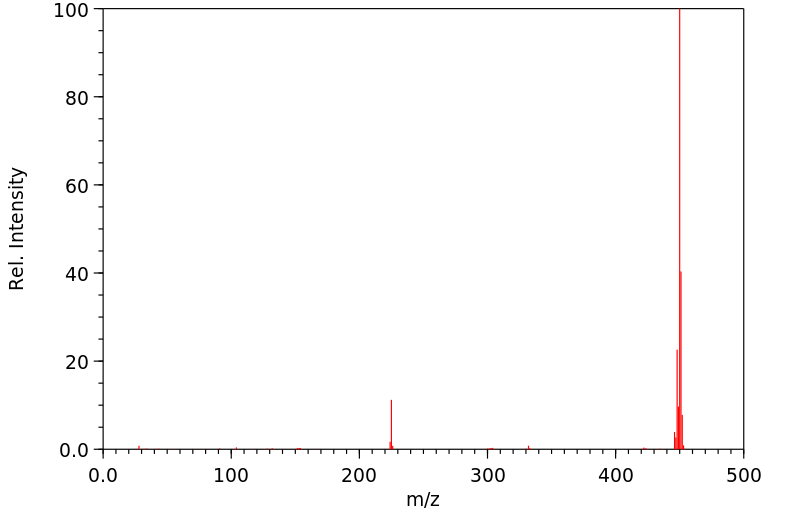
十环烯 | 191-48-0
中文名称
十环烯
中文别名
十环芳烃;环十轮烯
英文名称
decacyclene
英文别名
decacyclo[24.7.1.14,8.115,19.02,25.03,13.014,24.030,34.012,36.023,35]hexatriaconta-1(33),2,4,6,8(36),9,11,13,15,17,19(35),20,22,24,26,28,30(34),31-octadecaene
CAS
191-48-0
化学式
C36H18
mdl
——
分子量
450.539
InChiKey
CUIWZLHUNCCYBL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>300 °C(lit.)
-
密度:1.467±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,也没有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):9.7
-
重原子数:36
-
可旋转键数:0
-
环数:10.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
-
安全说明:S22,S24/25
-
储存条件:请将贮藏器密封并存放在阴凉、干燥处,同时确保工作环境有良好的通风或排气设施。
SDS
| Name: | Decacyclene 95% Material Safety Data Sheet |
| Synonym: | Diacenaphtho1,2-J:1',2'-L-Fluoranthene |
| CAS: | 191-48-0 |
Synonym:Diacenaphtho1,2-J:1',2'-L-Fluoranthene
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 191-48-0 | Diacenaphtho1,2-J:1',2'-L-Fluoranthene | 95 | 205-889-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 191-48-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: brown green
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: > 325 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C36H18
Molecular Weight: 450.53
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 191-48-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diacenaphtho1,2-J:1',2'-L-Fluoranthene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 191-48-0: No information available.
Canada
CAS# 191-48-0 is listed on Canada's NDSL List.
CAS# 191-48-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 191-48-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:用作化工原料,并广泛应用于测定大气污染和制备染料。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— circumtrindene —— C36H12 444.491
反应信息
-
作为反应物:描述:十环烯 在 palladium on activated charcoal 作用下, 反应 3.0h, 以4.3%的产率得到参考文献:名称:C36H12富勒烯亚基的合成和表征摘要:我们现在很高兴地报告包含 60% C{sub 60} 球的富勒烯片段的首次合成和表征:triacenaphthotriphenylene, 1 (C{sub 36}H{sub 12})。这项工作最令人欣慰的一个方面是,这种新的富勒烯片段可以通过“十环烯”2 的快速真空热解 (FVP) 一步制备,这种化合物已为化学家已知 100 多年,并已商业化。今天以公斤的数量提供。我们相信这里描述的高温化学与在富含燃料的火焰中运行的化学有着重要的关系,其中观察到富勒烯的产生。其他几种多环芳烃在 1200-1300{°C}C 范围内的新化学转化也已被发现并将很快报告。19 个参考文献,2 个无花果。DOI:10.1021/ja9621511
-
作为产物:参考文献:名称:酸催化环酮的醛醇缩合三聚反应制得的三环化苯衍生物。方法论发展与机制洞察摘要:为了阐明这一古老的反应的内在作用,已阐明了有助于醛醇三环化成功的几个因素。4,7-二叔丁基ac啶酮(11)作为机械探针分子的使用引起了有关温度,溶剂和溶解度影响的有趣发现。可极化且有点极性的溶剂,例如邻二氯苯(ODCB),对于所检测的芳族酮效果最佳。发现某些布朗斯台德酸在路易斯酸中作为非质子溶剂中茚满酮(2)的原型羟醛环三聚反应的催化剂效果更好,并且对p K a的依赖性强。观察到50%的酸。已显示使用对甲苯磺酸一水合物的标准化方案在许多测试案例中均能很好地工作。DOI:10.1021/jo070080q
文献信息
-
Palladium-catalyzed Trimerization of Strained Cycloalkynes: Synthesis of Decacyclene作者:Beatriz Iglesias、Diego Peña、Dolores Pérez、Enrique Guitián、Luis CastedoDOI:10.1055/s-2002-20473日期:——Palladium-catalyzed cyclotrimerization was applied to three strained cycloalkynes. Pd(PPh 3 ) 4 and Pt(PPh 3 ) 4 -catalyzed cyclotrimerizations of cyclohexyne (2) afforded dodecahydrotriphenylene (3) in 64% and 62% yields, respectively, but subjecting cyclopentyne to the same conditions failed to afford isolable amounts of the cyclotrimer. Finally, decacyclene (15), a putative C 6 0 -fullerene precursor
-
Circumtrindene: A Geodesic Dome of Molecular Dimensions. Rational Synthesis of 60 of C<sub>60</sub><sup>1</sup>作者:Ronald B. M. Ansems、Lawrence T. ScottDOI:10.1021/ja993028n日期:2000.3.1The synthetic strategy demonstrated here, which has improved the yield of circumtrindene (1) by more than 2 orders of magnitude, should be applicable to the rational synthesis of larger geodesic polyarenes, both open (bowls, baskets, tubes, etc.) and closed (fullerenes).
-
Novel Syntheses of Decacyclene by Deoxygenating Cyclotrimerisation of Acenaphthenequinone with Zero-valent Titanium or Phosphorus Pentasulfide作者:Klaus Zimmermann、Matthias HaenelDOI:10.1055/s-1997-3209日期:1997.5Decacylene (2) was obtained in 15-21% yield by reaction of acenaphthenequinone (6) with bis(η6-biphenyl)titanium(0) (9) in toluene or diglyme at 110°C and in 18% yield by reaction of 6 with phosphorus pentasulfide in boiling toluene. The new reactions are used to attempt the conversion of 3,8-dibromoacenaphthenequinone (7) to 3,4,9,10,15,16-hexabromodecacyclene (3) which is considered to serve as a suitable precursor for the bowl-shaped polycyclic aromatic hydrocarbon C36H12 (1).
-
Tridecacyclene: A Cyclic Tetramer of Acenaphthylene作者:Daniel P. Sumy、Nicholas J. Dodge、Chloe M. Harrison、Aaron D. Finke、Adam C. WhalleyDOI:10.1002/chem.201600165日期:2016.3.24the more planar decacyclene. The synthesized compound crystallizes into a unique packing structure with significant π‐stacking observed between adjacent molecules. Furthermore, due to its saddle‐like shape, the cyclic tetramer is able to form shape‐complementary interactions between its concave surface and the convex outer surface of buckminsterfullerene to generate cocrystalline supramolecular assemblies
-
2,5,8,11,14,17-Hexa-t-butyldecacyclene and 1,7,13-/1,6,12-tri-t-butyldecacyclene: Possible precursors for bowl-shaped polycyclic arenes作者:Klaus Zimmermann、Richard Goddard、Carl Krüger、Matthias W. HaenelDOI:10.1016/0040-4039(96)01933-8日期:1996.11Decacyclene (2) was converted into 2,5,8,11,14,17-hexa-t-butyldecacyclene (4) by Friedel-Crafts alkylation using chloride in 1,2-dichlorobenzene. Dehydrogenating cyclotrimerization of 5-t-butylacenaphthene by reaction with elemental sulfur resulted in 1,7,13- and 1,6,12-tri-t-butyldecacyclene () in the expected statistical 1:3 isomeric ratio. Single crystal X-ray structure analysis showed 4 to possess
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮