反式-2,2-二甲基-3-己烯 | 690-93-7
中文名称
反式-2,2-二甲基-3-己烯
中文别名
——
英文名称
(E)-2,2-dimethyl-3-hexene
英文别名
trans-2,2-dimethyl-3-hexene;trans-2,2-Dimethyl-hexen-(3);(E)-2,2-Dimethyl-3-hexen;trans-5,5-dimethyl-3-hexene;2,2-dimethyl-3-hexene;2.2-Dimethyl-trans-hexen-(3);2,2-Dimethyl-trans-3-hexen;trans-2,2-Dimethyl-3-hexen;2,2-Dimethyl-3-hexen;3-Hexene, 2,2-dimethyl-;(E)-2,2-dimethylhex-3-ene
CAS
690-93-7
化学式
C8H16
mdl
——
分子量
112.215
InChiKey
JPLZSSHKQZJYTJ-VOTSOKGWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-103.01°C (estimate)
-
沸点:100.85°C
-
密度:0.6995
-
保留指数:709;710.5;710;710
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 顺-2,2-二甲基-3-己烯 cis-2,2-dimethyl-3-hexene 690-92-6 C8H16 112.215 (Z)-5,5-二甲基己-2-烯 cis-5,5-Dimethyl-hexen-(2) 39761-61-0 C8H16 112.215 —— trans-5,5-dimethyl-2-hexene 39782-43-9 C8H16 112.215
反应信息
-
作为反应物:描述:反式-2,2-二甲基-3-己烯 在 platinum on activated charcoal 作用下, 生成 2,2-二甲基己烷参考文献:名称:Kasanskii et al., Doklady Akademii Nauk SSSR, 1950, vol. 71, p. 477摘要:DOI:
-
作为产物:描述:参考文献:名称:多重金属-碳键。43. 特性良好的高活性无路易斯酸烯烃复分解催化剂摘要:制备和活性 d'un complexe de W de type W(CH-t-Bu)(NR)(OR') 2 , OR'=OC(Me)(CF 3 ) 2DOI:10.1021/ja00270a056
文献信息
-
Efficient Epoxidation of Alkenes with Aqueous Hydrogen Peroxide Catalyzed by Methyltrioxorhenium and 3-Cyanopyridine作者:Hans Adolfsson、Christophe Copéret、Jay P. Chiang、Andrei K. YudinDOI:10.1021/jo005623+日期:2000.12.1The epoxidation of alkenes with 30% aqueous hydrogen peroxide is catalyzed efficiently by methyltrioxorhenium (MTO) in the presence of pyridine additives. The addition of 1-10 mol % of 3-cyanopyridine increases the system's efficiency for terminal and trans-disubstituted alkenes resulting in high isolated yields of the corresponding epoxides. The system allows for epoxidation of alkenes with various
-
Activation Parameters for the Epoxidation of Substitutedcis/trans Pairs of 1,2-Dialkylalkenes by Dimethyldioxirane作者:Brian S. Crow、W. Rucks Winkeljohn、Angela Navarro-Eisenstein、Elba Michelena-Baez、Paul J. Franklin、Pedro C. Vasquez、Al BaumstarkDOI:10.1002/ejoc.200600427日期:2006.10the corresponding cis isomers. The ΔH‡ terms mirrored trends observed in ΔG‡ because ΔS‡ terms for all ten of the compounds were roughly identical. The ΔΔG‡ values, a comparison of the trans to the cis isomer data, yielded positive values of 1.2 to 1.8 kcal/mol for the five sets of data and appeared to be dependent on relative steric interactions. The experimental activation parameter data, consistent报告了二甲基二环氧乙烷 (1) 与五个顺式/反式烯烃对反应的第一个活化参数数据。顺式-1,2-二烷基烯烃的环氧化(2顺式:R1 = Me,R2 = iPr;3顺式:R1 = Me,R2 = tBu;4顺式:R1 = R2 = Et;5顺式:R1 = Et,R2 = iPr;6顺式:R1 = Et,R2 = tBu)和反式-1,2-二烷基烯烃(2trans:R1 = Me,R2 = iPr;3trans:R1 = Me,R2 = tBu;4trans:R1 = R2 = Et;5trans:R1 = Et, R2 = iPr; 6trans: R1 = Et, R2 = tBu) 通过 1 产生相应的环氧化物,定量和立体定向,作为唯一可观察的产物。使用 Arrhenius 方法确定五对烯烃,2cis-6cis 和 2trans-6trans,1 环氧化的活化参数。在较低温度下观察到顺式与反式
-
The direct arylation of allylic sp3 C–H bonds via organic and photoredox catalysis作者:James D. Cuthbertson、David W. C. MacMillanDOI:10.1038/nature14255日期:2015.3.5particular, the direct arylation of non-functionalized allylic systems would enable access to a series of known pharmacophores (molecular features responsible for a drug’s action), though a general solution to this long-standing challenge remains elusive. Here we report the use of both photoredox and organic catalysis to accomplish a mild, broadly effective direct allylic C–H arylation. This C–C bond forming未活化的 sp3 C-H 键的直接功能化仍然是合成有机化学家面临的最具挑战性的问题之一。这种转变的吸引力来自于它们通过简单和其他惰性结构单元的耦合促进复杂有机分子构建的能力,而不会引入无关的官能团。尽管最近做出了显着的努力,但事实证明,为 sp3 C-H 键参与 C-C 键形成反应建立一般和温和的策略是困难的。在此背景下,发现能够以催化方式直接使烯丙基甲基、亚甲基和次甲基碳官能化的化学转化是当务之急。尽管烯丙基 C-H 键的直接氧化和胺化方案(即,C-H 键,其中相邻的碳参与 C = C 键)已经广泛建立,烯丙基底物在 C-C 键形成反应中的参与迄今为止需要使用预官能化的偶联伙伴。特别是,非功能化烯丙基系统的直接芳基化将能够获得一系列已知的药效团(负责药物作用的分子特征),尽管解决这一长期挑战的通用解决方案仍然难以捉摸。在这里,我们报告了使用光氧化还原和有机催化来完成温和、广泛有效的直接烯丙基
-
Stereochemistry and regiochemistry of the addition of lodonium nitrate to alkenes作者:J. William Lown、Alummoottil V. JoshuaDOI:10.1039/p19730002680日期:——The stereochemistry of the products was confirmed by relating them chemically with known compounds. Addition to the less hindered (Z)-[β-2H]styrene is also stereospecific, eliminating the possibility of restricted rotation during addition. Ring closure by neighbouring sulphur in the addition of iodonium nitrate to 1-allyl-3,3-diethylthiourea affords a thiazole. The failure to obtain addition and phenyl
-
A stereo- and regio-specific addition of η3-trimethylsilylallyltitanium compound with aldehydes. A facile and stereocontrolled synthesis of E- and Z-terminal dienes作者:Fumie Sato、Yoshito Suzuki、Masao SatoDOI:10.1016/s0040-4039(00)85661-0日期:1982.1η3-Trimethylsilyallyltitanium compound, (η5-C5H5)2Ti(η3-1-trimethylsilylallyl), reacts with aldehydes to give (±)-(R,S)-3-trimethylsilyl-4-hydroxy-1-alkenes in excellent yields, which can be deoxysilylated to either E- or Z-1,3-dienes.
表征谱图
-
氢谱1HNMR
-
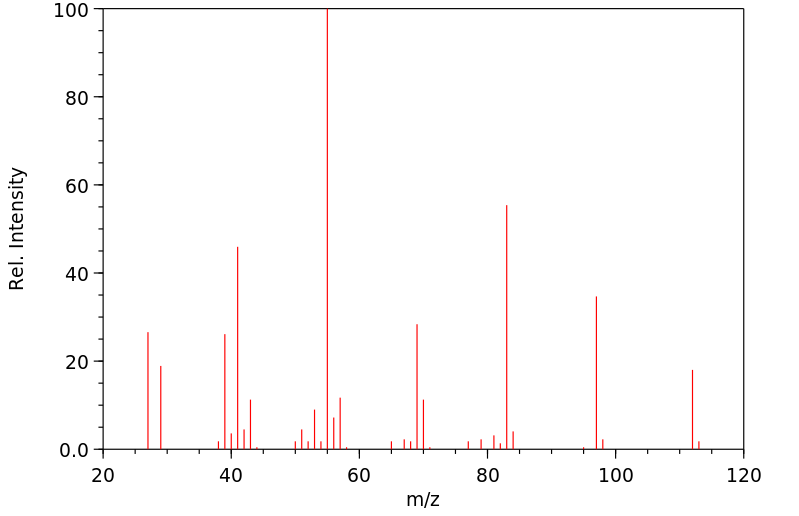
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-