叔丁基二甲胺 | 918-02-5
中文名称
叔丁基二甲胺
中文别名
N,N-二甲基叔丁胺;叔丁基二甲基胺
英文名称
N,N-dimethyl-tert-butylamine
英文别名
tert-butyl-di-methylamine;tert-butoxy-bis(dimethylamino)methane;N,N-dimethyl-t-butylamine;tert-butyl(dimethyl)amine;tert-butyldimethylamine;dimethyl-tert-butylamine;2-Propanamine, N,N,2-trimethyl-;N,N,2-trimethylpropan-2-amine
CAS
918-02-5
化学式
C6H15N
mdl
MFCD03452796
分子量
101.192
InChiKey
OXQMIXBVXHWDPX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-90 °C
-
沸点:89.6-89.9 °C (740 mmHg)
-
密度:0.739
-
闪点:-5 °C
-
保留指数:693
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:CORROSIVE
-
安全说明:S16,S26,S36/37/39,S45
-
危险品标志:C
-
危险类别码:R34
-
储存条件:密封贮藏,存放在阴凉、干燥处,远离热源、火源和火焰,避免靠近易燃易爆炸区域。
SDS
| Name: | tert-Butyldimethylamine 98% Material Safety Data Sheet |
| Synonym: | N,N-Dimethyl-tert-butylamin |
| CAS: | 918-02-5 |
Synonym:N,N-Dimethyl-tert-butylamin
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 918-02-5 | tert-Butyldimethylamine | 98% | unlisted |
Risk Phrases: 11 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Highly flammable. Causes burns.
Potential Health Effects
Eye:
Causes eye burns.
Skin:
Causes skin burns.
Ingestion:
Causes gastrointestinal tract burns.
Inhalation:
Causes chemical burns to the respiratory tract.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors can travel to a source of ignition and flash back. Will burn if involved in a fire. Flammable liquid and vapor.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide. Water may be ineffective.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Remove all sources of ignition.
Use a spark-proof tool.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a cool, dry place. Store in a tightly closed container. Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 918-02-5: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 89.6 - 89.9 deg C @740mmHg
Freezing/Melting Point: -90 deg C
Autoignition Temperature: Not available.
Flash Point: -5 deg C ( 23.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 0.739
Molecular Formula: C6H15N
Molecular Weight: 101.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 918-02-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
tert-Butyldimethylamine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: AMINES, FLAMMABLE, CORROSIVE, N.O.S.*
Hazard Class: 3 (8)
UN Number: 2733
Packing Group: II
IMO
Shipping Name: AMINES, FLAMMABLE, CORROSIVE, N.O.S.
Hazard Class: 3 (8)
UN Number: 2733
Packing Group: II
RID/ADR
Shipping Name: AMINES, FLAMMABLE, CORROSIVE, N.O.S.
Hazard Class: 3 (8)
UN Number: 2733
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: F C
Risk Phrases:
R 11 Highly flammable.
R 34 Causes burns.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 918-02-5: No information available.
Canada
CAS# 918-02-5 is listed on Canada's NDSL List.
CAS# 918-02-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 918-02-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:叔丁基二甲胺 在 过氧碳酸钠 、 sodium salt of 2,4-dichloro-6-hydroxy-1,3,5-triazine aluminum oxide 、 silica gel 、 potassium carbonate 作用下, 以 氯仿 为溶剂, 以93%的产率得到N,2-二甲基丙烷-2-胺参考文献:名称:在便利的柱色谱样设置中,使用固体试剂对叔胺进行一般的,选择性的,高产率的N-脱甲基步骤。摘要:[反应:请参见文本]传统的制备型色谱柱可用于实现叔N-甲基胺和生物碱的定量N-去甲基化。填充物是至关重要的部分,在三个反应区中装有不同的固体试剂。将母体化合物装到色谱柱上,几分钟后,纯净的N-脱甲基仲胺离开色谱柱。DOI:10.1021/ol036319g
-
作为产物:参考文献:名称:脱芳构的PN 3 P *-氢化镍夹钳复合物对CO 2还原的不同催化活性†摘要:使用氧化加成法制备了脱芳香化的PN 3 P *-氢化镍配合物。首次实现了镍催化的CO 2加氢硅烷化为甲醇,其转化率空前。此类PN 3 P *-氢化镍氢化物证明了胺与CO 2的选择性甲基化和甲酰化,突出了其在CO 2还原中的多功能性。DOI:10.1039/c8cc05948a
-
作为试剂:参考文献:名称:Phenyl Chloro(thionoformate): a New Dealkylating Agent of Tertiary Amines摘要:苯基氯代(硫代甲酸酯)在20°下与无障碍的三级脂肪胺迅速反应,生成硫代氨酸酯和烷基氯化物。二烷基环己胺出奇地迅速反应,主要生成环己烯。硫代氨酸酯经过甲基硫酸二甲酯处理后,再用水水解转化为二级胺盐。在氨基中,反应速率和烷基团裂解选择性被发现优于或与以前报道的氯代甲酸酯相媲美。DOI:10.1071/ch98147
文献信息
-
HETEROBICYCLIC COMPOUNDS申请人:Amgen Inc.公开号:US20130225552A1公开(公告)日:2013-08-29Heterobicyclic compounds of Formula (I): or a pharmaceutically-acceptable salt, tautomer, or stereoisomer thereof, as defined in the specification, and compositions containing them, and processes for preparing such compounds. Provided herein also are methods of treating disorders or diseases treatable by inhibition of PDE10, such as obesity, non-insulin dependent diabetes, schizophrenia, bipolar disorder, obsessive-compulsive disorder, Huntington's Disease, and the like.
-
Novel Thiazole Inhibitors of Fructose 1,6-Bishosphatase申请人:Dang Qun公开号:US20070225259A1公开(公告)日:2007-09-27Compounds of Formula I, their prodrugs and salts, their preparation and their uses are described.公式I的化合物,它们的前药和盐,它们的制备以及它们的用途被描述了。
-
[EN] IMIDAZOTHIADIAZOLE AND IMIDAZOPYRAZINE DERIVATIVES AS PROTEASE ACTIVATED RECEPTOR 4 (PAR4) INHIBITORS FOR TREATING PLATELET AGGREGATION<br/>[FR] DÉRIVÉS D'IMIDAZOTHIADIAZOLE ET D'IMIDAZOPYRAZINE UTILISÉS COMME INHIBITEURS DU RÉCEPTEUR 4 ACTIVÉ PAR UNE PROTÉASE (PAR4) POUR LE TRAITEMENT DE L'AGRÉGATION PLAQUETTAIRE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2013163279A1公开(公告)日:2013-10-31The present invention provides thiazole compounds of Formula I wherein W, Y, R0, R2, R4, R5, R6, R7, X1, X2, X3 and X4 are as defined herein, or a stereoisomer, tautomer, pharmaceutically acceptable salt, prodrug ester or solvate form thereof, wherein all of the variables are as defined herein. These compounds are inhibitors of platelet aggregation and thus can be used as medicaments for treating or preventing thromboembolic disorders.
-
CONJUGATES OF CEREBLON BINDING COMPOUNDS AND G12C MUTANT KRAS, HRAS OR NRAS PROTEIN MODULATING COMPOUNDS AND METHODS OF USE THEREOF申请人:Araxes Pharma LLC公开号:US20180015087A1公开(公告)日:2018-01-18Conjugates of a cereblon-binding compound and compounds having modulatory activity against G12C mutant KRAS, HRAS or NRAS G12C proteins are provided. Methods associated with preparation and use of such conjugates, pharmaceutical compositions comprising such conjugates and methods to modulate the activity of G12C mutant KRAS, HRAS or NRAS G12C proteins for treatment of disorders, such as cancer, are also provided.提供了与谷氨酰脑结合化合物和具有调节活性对抗G12C突变KRAS、HRAS或NRAS G12C蛋白的化合物的共轭物。还提供了与制备和使用这种共轭物相关的方法,包括含有这种共轭物的药物组合物以及调节G12C突变KRAS、HRAS或NRAS G12C蛋白活性的方法,用于治疗癌症等疾病。
-
Reductive methylation of primary and secondary amines and amino acids by aqueous formaldehyde and zinc作者:Renato A. da Silva、Idália H.S. Estevam、Lothar W. BieberDOI:10.1016/j.tetlet.2007.08.092日期:2007.10Amines can be methylated when treated with formaldehyde and zinc in aqueous medium. Selective mono- or dimethylation can be achieved by proper choice of pH, stoichiometry and reaction time. This method can also be applied for amino acids.
表征谱图
-
氢谱1HNMR
-
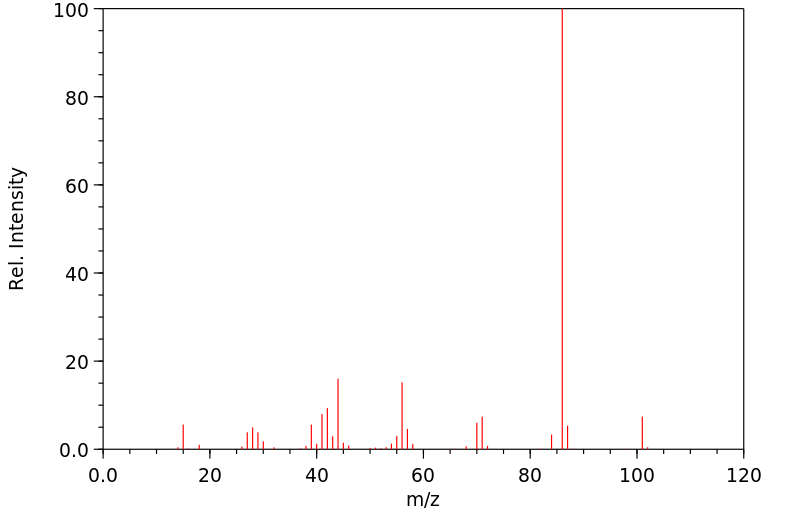
质谱MS
-
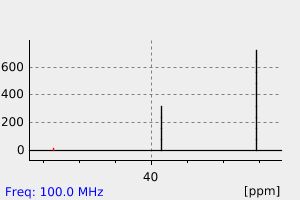
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷