对羟基苯甲酸异戊酯 | 6521-30-8
中文名称
对羟基苯甲酸异戊酯
中文别名
4-羟基苯甲酸异戊酯
英文名称
isopentylparaben
英文别名
Isoamyl p-hydroxybenzoate;Isopentyl 4-hydroxybenzoate;3-methylbutyl 4-hydroxybenzoate
CAS
6521-30-8
化学式
C12H16O3
mdl
MFCD00017502
分子量
208.257
InChiKey
KSHVDKDQYBNSAN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:62 °C
-
沸点:242 °C
-
密度:1.083±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
-
保留指数:1822
-
稳定性/保质期:
存在于烟叶中。
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.416
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
储存条件:室温且干燥
SDS
Isoamyl 4-Hydroxybenzoate Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Isoamyl 4-Hydroxybenzoate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
Skin sensitization Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
May cause an allergic skin reaction
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Contaminated work clothing should not be allowed out of the workplace.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation or rash occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Isoamyl 4-Hydroxybenzoate
Percent: >98.0%(GC)(T)
6521-30-8
CAS Number:
Synonyms: 4-Hydroxybenzoic Acid Isoamyl Ester , Isoamylparaben , Isopentyl 4-
Hydroxybenzoate
Chemical Formula: C12H16O3
Isoamyl 4-Hydroxybenzoate
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
White - Almost white
Colour:
Isoamyl 4-Hydroxybenzoate
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odour: No data available
pH: No data available
Melting point/freezing point:62°C
Boiling point/range: 242°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Isoamyl 4-Hydroxybenzoate
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Isoamyl 4-Hydroxybenzoate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
Skin sensitization Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
May cause an allergic skin reaction
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Contaminated work clothing should not be allowed out of the workplace.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation or rash occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Isoamyl 4-Hydroxybenzoate
Percent: >98.0%(GC)(T)
6521-30-8
CAS Number:
Synonyms: 4-Hydroxybenzoic Acid Isoamyl Ester , Isoamylparaben , Isopentyl 4-
Hydroxybenzoate
Chemical Formula: C12H16O3
Isoamyl 4-Hydroxybenzoate
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
White - Almost white
Colour:
Isoamyl 4-Hydroxybenzoate
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odour: No data available
pH: No data available
Melting point/freezing point:62°C
Boiling point/range: 242°C
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Isoamyl 4-Hydroxybenzoate
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:Synthetische Studien über die Beziehung zwischen chemischer Konstitution und antimikrober Wirkung XII. 3-Nitro- und 3-Amino-4-oxy bzw. alkoxybenzoesäureester摘要:DOI:10.1002/ardp.19342721022
-
作为产物:描述:4-((isopentyloxy)carbonyl)-N,N,N-trimethylbenzenaminium trifluoromethanesulfonate 在 caesium carbonate 、 苯甲醛肟 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 4.0h, 以98%的产率得到对羟基苯甲酸异戊酯参考文献:名称:温和条件下芳基铵盐合成酚类摘要:开发了一种在温和条件下由缺电子芳基铵盐或杂芳基铵盐合成酚类的通用方法。苯甲醛肟、乙酰氧肟酸和盐酸羟胺分别作为氢氧化物替代物进行了研究。使用这些氢氧化物替代物,可以以 20-98% 的收率制备一系列酚类。DOI:10.1021/acs.joc.2c01133
文献信息
-
Metabolism and Pharmacokinetics of Oxazaphosphorines作者:Alan V. Boddy、S. Murray YuleDOI:10.2165/00003088-200038040-00001日期:2000.4The 2 most commonly used oxazaphosphorines are cyclophosphamide and ifosfamide, although other bifunctional mustard analogues continue to be investigated. The pharmacology of these agents is determined by their metabolism, since the parent drug is relatively inactive. For cyclophosphamide, elimination of the parent compound is by activation to the 4-hydroxy metabolite, although other minor pathways of inactivation also play a role. Ifosfamide is inactivated to a greater degree by dechloroethylation reactions. More robust assay methods for the 4-hydroxy metabolites may reveal more about the clinical pharmacology of these drugs, but at present the best pharmacodynamic data indicate an inverse relationship between plasma concentration of parent drug and either toxicity or antitumour effect. The metabolism of cyclophosphamide is of particular relevance in the application of high dose chemotherapy. The activation pathway of metabolism is saturable, such that at higher doses (greater than 2 to 4 g/m2) a greater proportion of the drug is eliminated as inactive metabolites. However, both cyclophosphamide and ifosfamide also act to induce their own metabolism. Since most high dose regimens require a continuous infusion or divided doses over several days, saturation of metabolism may be compensated for, in part, by auto-induction. Although a quantitative distinction may be made between the cytochrome P450 isoforms responsible for the activating 4-hydroxylation reaction and those which mediate the dechloroethylation reactions, selective induction of the activation pathway, or inhibition of the inactivating pathway, has not been demonstrated clinically. Mathematical models to describe and predict the relative contributions of saturation and autoinduction to the net activation of cyclophosphamide have been developed. However, these require careful validation and may not be applicable outside the exact regimen in which they were derived. A further complication is the chiral nature of these 2 drugs, with some suggestion that one enantiomer may have a favourable profile of metabolism over the other. That the oxazaphosphorines continue to be the subject of intensive investigation over 30 years after their introduction into clinical practice is partly because of their antitumour activity. Further advances in analytical and molecular pharmacological techniques may further optimise their use and allow rational design of more selective analogues.最常用的两种噁唑磷酰胺类药物是环磷酰胺和异环磷酰胺,尽管其他双功能烷化剂仍在研究中。这些药物的药理学特性由其代谢途径决定,因为母体药物相对不活跃。环磷酰胺通过激活生成4-羟基代谢物来消除母体化合物,尽管其他次要的失活途径也起作用。异环磷酰胺通过脱氯乙基化反应更大幅度地失活。更强大的4-羟基代谢物检测方法可能揭示这些药物的临床药理学更多信息,但目前最佳药效学数据显示,母体药物血浆浓度的逆向关系与毒性或抗肿瘤效应相关。环磷酰胺的代谢在应用高剂量化疗时尤为相关。代谢激活途径是可饱和的,因此在高剂量(大于2至4 g/m²)下,药物以失活代谢物的形式排出的比例更大。然而,环磷酰胺和异环磷酰胺都能诱导自身代谢。由于大多数高剂量方案需要连续输注或几天的分次给药,代谢饱和可通过自诱导部分得到补偿。尽管可以对负责激活4-羟基化反应的细胞色素P450同工酶和介导脱氯乙基化反应的同工酶进行定量区分,但临床上尚未证明选择性诱导激活途径或抑制失活途径的能力。描述和预测饱和和自诱导对环磷酰胺净激活相对贡献的数学模型已开发出来。然而,这些模型需要仔细验证,并且可能不适用于它们所衍生的给药方案之外。另一个复杂之处在于这两种药物的手性本质,有迹象表明一种对映体可能比另一种具有更有利的代谢特征。由于其抗肿瘤活性,噁唑磷酰胺类药物在引入临床实践30多年后,仍然是广泛研究的主题。进一步发展分析和分子药理学技术可能进一步优化它们的使用,并允许设计更具选择性的类似物。
-
HIGHLY PHOTO-STABLE BIS-TRIAZOLE FLUOROPHORES申请人:NITTO DENKO CORPORATION公开号:US20180002337A1公开(公告)日:2018-01-04This disclosure is related to photo-stable chromophores which are useful in various applications. Chromophores disclosed herein include a bis-triazole core, two electron-donors at C-4 and C-8, and two groups derived from pentaerythritol (R═OR 5 ) or 1,1,1-tris(hydroxymethyl)methane (R═H) at N-2 and N-6. Such structures have been proven to have greater then five times higher photo-stability than their analogs with simpler alkyl groups at N-2 and N-6.
-
METHOD FOR PRODUCING FLUOROVINYL ETHER COMPOUND申请人:DAIKIN INDUSTRIES, LTD.公开号:US20210284593A1公开(公告)日:2021-09-16An object of the present invention is to provide, for example, a novel method for synthesizing a fluorovinyl ether compound from a fluorine-containing vinyl compound. This problem is solved by a method for producing a compound represented by formula (1): wherein R a1 is a hydrogen atom, a halogeno group, an alkyl group, a fluoroalkyl group, or an aromatic group optionally having one or more substituents, Rf is a fluoro group or a perfluoroalkyl group, R a2 is a hydrogen atom, a halogeno group, an alkyl group, a fluoroalkyl group, or an aromatic group optionally having one or more substituents, or (i) R a1 and R a2 , (ii) R a1 and Rf, or (iii) Rf and R a2 may be linked to each other, R b1 is R S , R b2 is a hydrogen atom or R S , R b3 is a hydrogen atom or R S , or two or three of R b1 , R b2 , and R b3 , taken together with the adjacent carbon atom, may form a ring optionally having one or more substituents, and R S , in each occurrence, is the same or different and represents a hydrocarbon group optionally having one or more substituents, the method comprising step A of reacting a compound represented by formula (2): wherein R x is a leaving group, and other symbols are as defined above, with a compound represented by formula ( 3 ): wherein the symbols in the formula are as defined above, in the presence of a transition metal catalyst.本发明的一个目标是提供一种从含氟乙烯化合物合成氟乙烯醚化合物的新方法。这个问题通过以下方法解决:生产一个由以下式表示的化合物的方法(1):其中Ra1是氢原子、卤素基团、烷基基团、氟烷基基团或可能具有一个或多个取代基的芳香基团,Rf是氟基团或全氟烷基基团,Ra2是氢原子、卤素基团、烷基基团、氟烷基基团或可能具有一个或多个取代基的芳香基团,或(i) Ra1和Ra2、(ii) Ra1和Rf或(iii) Rf和Ra2可能连接在一起,Rb1是RS,Rb2是氢原子或RS,Rb3是氢原子或RS,或者Rb1、Rb2和Rb3中的两个或三个,与相邻的碳原子一起,可以形成一个可能具有一个或多个取代基的环,RS在每次出现时相同或不同,表示一个可能具有一个或多个取代基的碳氢基团,该方法包括以下步骤:步骤A,将由以下式表示的化合物(2):其中Rxis是一个离去基团,和上述其他符号的定义相同,与由以下式表示的化合物(3):其中式中的符号与上述定义相同,在过渡金属催化剂存在下反应。
-
CRYSTAL OF THIAZOLIDINEDIONE COMPOUND, AND PROCESS FOR PRODUCTION THEREOF申请人:Daiichi Sankyo Company, Limited公开号:EP2305673A1公开(公告)日:2011-04-06Thiazolidinedione compound crystalline forms useful as a bulk material for the manufacture of a peroxisome proliferator-activated receptor (PPAR) γ activator or an anticancer pharmaceutical composition are provided, which are hydrate crystalline forms of 5-(4-[6-(4-amino-3,5-dimethylphenoxy)-l-methyl-l-H-benzimi dazol-2-yl]methoxy}benzyl)-l,3-thiazolidine-2,4-dione represented by Formula (I) below and of its dihydrochloride.
-
Crystalline forms of thiazolidinedione compound and its manufacturing method申请人:Kajino Hisaki公开号:US20110046388A1公开(公告)日:2011-02-24It is an object of the present invention to provide the crystalline form of a thiazolidinedione compound, which is effective as a pharmaceutical ingredient for manufacturing a peroxisome proliferator-activated receptor (PPAR) activator and an anticancer pharmaceutical composition. The present invention relates to a crystalline form of a hydrate of 5-(4-[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1-H benzimidazol-2-yl]methoxy}benzyl)-1,3-thiazolidine-2,4-dione dihydrochloride represented by the following formula (I). [Formula 1]
表征谱图
-
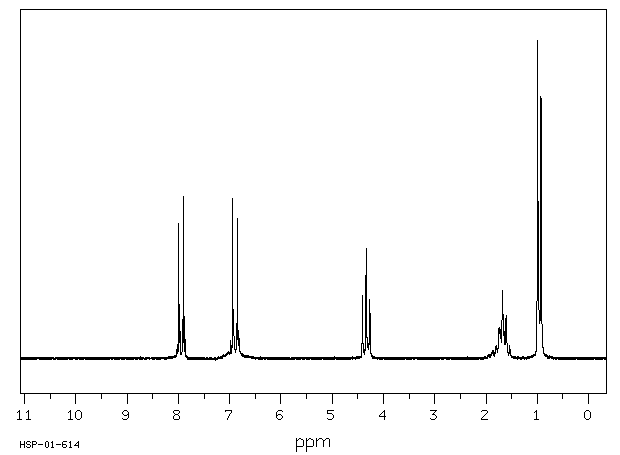
氢谱1HNMR
-
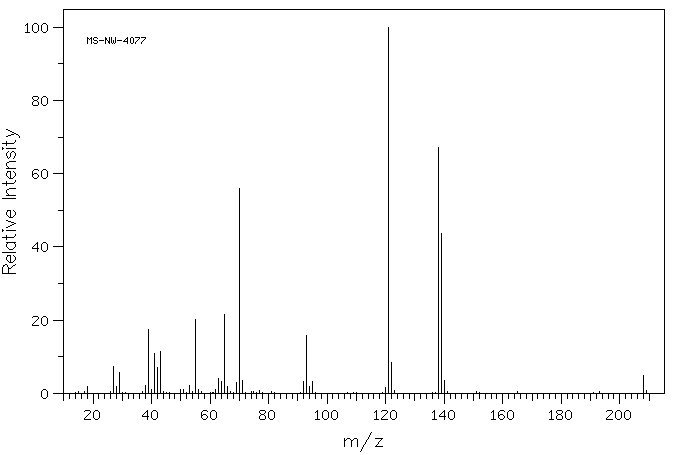
质谱MS
-
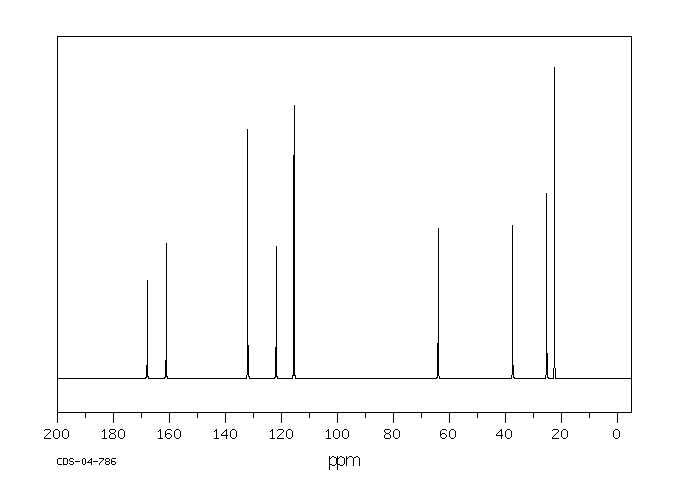
碳谱13CNMR
-
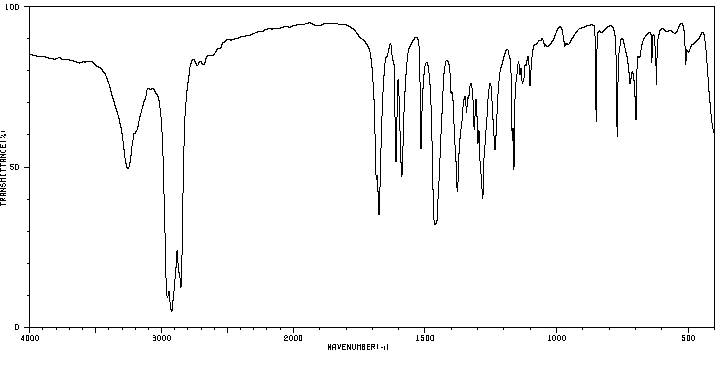
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫