(4-甲酰基-2-甲氧基苯氧基)乙酸甲酯 | 79317-30-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:345.1±27.0 °C(Predicted)
-
密度:1.208±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:16
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.272
-
拓扑面积:61.8
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2918990090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (4-甲酰基-2-甲氧基苯氧基)乙酸 (4-formyl-2-methoxyphenoxy)acetic acid 1660-19-1 C10H10O5 210.186 香草醛 vanillin 121-33-5 C8H8O3 152.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (4-Hydroxymethyl-2-methoxy-phenoxy)-acetic acid methyl ester 761409-39-6 C11H14O5 226.229 —— methyl 2-(4-(1,3-dioxolan-2-yl)-2-methoxyphenoxy)acetate 182345-41-1 C13H16O6 268.266 —— methyl 2-(4-hydroxy-2-methoxyphenoxy)acetate —— C10H12O5 212.202
反应信息
-
作为反应物:描述:(4-甲酰基-2-甲氧基苯氧基)乙酸甲酯 在 4-二甲氨基吡啶 、 水 、 对甲苯磺酸 、 N,N'-二环己基碳二亚胺 、 sodium hydroxide 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 甲醇 、 乙醇 、 二氯甲烷 、 苯 为溶剂, 反应 31.0h, 生成 3-[3-methoxy-4-(methoxymethoxy)phenyl]-2-{2-methoxy-4-[(E)-2-({2-[4-(tetrahydro-2H-pyran-2-yloxy)phenyl]ethyl}carbamoyl)eth-1-en-1-yl]phenoxy}-N-{2-[4-(tetrahydro-2H-pyran-2-yloxy)phenyl]ethyl}prop-2-enamide参考文献:名称:Total synthesis of cannabisin F摘要:
摘要 首次报道了一种从香草醛开始的实用八步合成木脂素类大麻素F的方法。这种合成策略应用了醛缩反应,然后是Wittig反应,以得到关键的8-O-4'-新木脂素中间体二酸。该二酸与N,O-保护的酪胺缩合,去保护后形成大麻素F。
DOI:10.2478/s11696-013-0449-y -
作为产物:描述:参考文献:名称:Total synthesis of cannabisin F摘要:
摘要 首次报道了一种从香草醛开始的实用八步合成木脂素类大麻素F的方法。这种合成策略应用了醛缩反应,然后是Wittig反应,以得到关键的8-O-4'-新木脂素中间体二酸。该二酸与N,O-保护的酪胺缩合,去保护后形成大麻素F。
DOI:10.2478/s11696-013-0449-y -
作为试剂:描述:香草醛 、 sodium;hydride 、 溴乙酸甲酯 、 水 在 四氢呋喃 、 乙酸乙酯 、 magnesium sulfate 、 (4-甲酰基-2-甲氧基苯氧基)乙酸甲酯 、 SiO2 、 ethyl acetate n-hexane 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 15.0h, 生成 (4-甲酰基-2-甲氧基苯氧基)乙酸甲酯参考文献:名称:4-and 5-alkynyloxindoles and 4-and 5-alkenyloxindoles摘要:本发明涉及4-和5-炔基氧吲哚以及4-和5-烯基氧吲哚,这些化合物能够抑制或调节蛋白激酶,特别是JNK蛋白激酶。本发明的化合物及其药学上可接受的盐和前药,可用作抗炎剂,特别适用于治疗类风湿关节炎。本发明还涉及含有上述化合物的制药组合物,使用这些化合物治疗和/或控制炎症的方法,特别是使用这些化合物治疗或控制类风湿关节炎,以及在制备本发明化合物中有用的中间体。公开号:US06313310B1
文献信息
-
Targeting Receptor Tyrosine Kinase VEGFR-2 in Hepatocellular Cancer: Rational Design, Synthesis and Biological Evaluation of 1,2-Disubstituted Benzimidazoles作者:Heba T. Abdel-Mohsen、Mona A. Abdullaziz、Ahmed M. El Kerdawy、Fatma A. F. Ragab、Keith J. Flanagan、Abeer E. E. Mahmoud、Mamdouh M. Ali、Hoda I. El Diwani、Mathias O. SengeDOI:10.3390/molecules25040770日期:——
In this study, a novel series of 1,2-disubstituted benzo[d]imidazoles was rationally designed as VEGFR-2 inhibitors targeting hepatocellular carcinoma. Our design strategy is two-fold; it aimed first at studying the effect of replacing the 5-methylfuryl moiety of the well-known antiangiogenic 2-furylbenzimidazoles with an isopropyl moiety on the VEGFR-2 inhibitory activity and the cytotoxic activity. Our second objective was to further optimize the structures of the benzimidazole derivatives through elongation of the side chains at their one-position for the design of more potent type II-like VEGFR-2 inhibitors. The designed 1,2-disubstituted benzimidazoles demonstrated potent cytotoxic activity against the HepG2 cell line, reaching IC50 = 1.98 μM in comparison to sorafenib (IC50 = 10.99 μM). In addition, the synthesized compounds revealed promising VEGFR-2 inhibitory activity in the HepG2 cell line, e.g., compounds 17a and 6 showed 82% and 80% inhibition, respectively, in comparison to sorafenib (% inhibition = 92%). Studying the effect of 17a on the HepG2 cell cycle demonstrated that 17a arrested the cell cycle at the G2/M phase and induced a dose-dependent apoptotic effect. Molecular docking studies of the synthesized 1,2-disubstituted benzimidazoles in the VEGFR-2 active site displayed their ability to accomplish the essential hydrogen bonding and hydrophobic interactions for optimum inhibitory activity.
在这项研究中,一系列新型的1,2-二取代苯并[d]咪唑被合理设计为靶向肝细胞癌的VEGFR-2抑制剂。我们的设计策略是双重的;首先旨在研究将众所周知的抗血管生成的2-呋喃基苯并咪唑的5-甲基呋喃基团替换为异丙基基团对VEGFR-2抑制活性和细胞毒活性的影响。我们的第二个目标是通过延长它们的一位点的侧链来进一步优化苯并咪唑衍生物的结构,设计更强效的类II型VEGFR-2抑制剂。设计的1,2-二取代苯并咪唑表现出强大的细胞毒活性,对HepG2细胞系的IC50 = 1.98 μM,而索拉非尼的IC50 = 10.99 μM。此外,合成的化合物在HepG2细胞系中显示出有希望的VEGFR-2抑制活性,例如,化合物17a和6分别显示出82%和80%的抑制作用,而索拉非尼的抑制率为92%。研究17a对HepG2细胞周期的影响表明,17a在G2/M期阻滞细胞周期并诱导剂量依赖的凋亡效应。对合成的1,2-二取代苯并咪唑在VEGFR-2活性位点的分子对接研究显示它们能够完成必要的氢键和疏水相互作用,以实现最佳的抑制活性。 -
Synthesis and pharmacological characterization of novel xanthine carboxylate amides as A2A adenosine receptor ligands exhibiting bronchospasmolytic activity作者:Rakesh Yadav、Ranju Bansal、Suman Rohilla、Sonja Kachler、Karl-Norbert KlotzDOI:10.1016/j.bioorg.2016.01.003日期:2016.4represent a new series of selective ligands of the adenosine A2A receptors exhibiting bronchospasmolytic activity. The effects of location of 8-phenyl substitutions on the adenosine receptor (AR) binding affinities of the newly synthesized xanthines have also been studied. The compounds displayed moderate to potent binding affinities toward various adenosine receptor subtypes when evaluated through radioligand本文所述的8-苯基-1,3-二甲基黄嘌呤的羧酸酰胺代表展现支气管痉挛活性的腺苷A 2A受体的一系列新的选择性配体。还研究了8-苯基取代位置对新合成的黄嘌呤腺苷受体(AR)结合亲和力的影响。通过放射配体结合研究评估时,这些化合物对各种腺苷受体亚型显示出中等至有效的结合亲和力。但是,大多数化合物显示出对A 2A亚型的最大亲和力,有些相对于所有其他亚型具有高选择性。黄嘌呤羧酸酰胺13b在对位具有二乙基氨基乙基氨基部分将8-苯基黄嘌呤支架的-位确定为最有效的A 2A腺苷受体配体,K i = 0.06μM。同样有效且高度A 2A选择性的是异香草醛衍生物16a和16d。另外,当在豚鼠中测试时,新合成的黄嘌呤衍生物显示出良好的体内支气管痉挛活性。
-
[EN] HYPOPHOSPHOROUS ACID DERIVATIVES HAVING ANTIHYPERALGIC ACTIVITY AND BIOLOGICAL APPLICATIONS THEREOF<br/>[FR] DÉRIVÉS DE L'ACIDE HYPOPHOSPHOREUX AYANT UNE ACTIVITÉ ANTIHYPERALGIQUE ET LEURS APPLICATIONS BIOLOGIQUES申请人:UNIV PARIS DESCARTES公开号:WO2012156931A1公开(公告)日:2012-11-22The invention relates to hypophosphorous acid derivatives of formula (I) wherein - X is H or OH, - R represents one or several radicals R1-R5, identical or different, two of R1-R5 optionally occupying the same position on the phenyl group, one to four of R1-R5 being H and the others being selected in the group comprising - 0-(CH2)n-COOH; - S-(CH2)n-COOH; -NH-(CH2)n-COOH; - 0-(CH,R') -COOH; -O- (CH2)n-OH; OR', -R' being a C1 -C3 alkyl radical;-OH; --COOH; halogen, particularly -F, - CI, -Br, -I, -CF3; -OCF3; -N02; -CH=CH-COOH; - -(CH2)n-COOH; O - (CH2)n- P03H2; O - (CF2)n- P03H2; O - (CH2)n- S03H; O - (CH2)n- CONHOH; O - (CH2)n-tetrazol; O - (CH2)n-hydroxyisoxazol - n = 1 to 5, preferably 1-3; said hypophosrous acid derivatives being diastereoisomers or enantiomers.该发明涉及式(I)的次磷酸衍生物,其中- X为H或OH,- R代表一个或几个基团R1-R5,相同或不同,R1-R5中的两个可以选择占据苯基上的同一位置,R1-R5中的一到四个为H,其余的选择自-0-(CH2)n-COOH;-S-( )n-COOH;-NH-( )n-COOH;-0-(CH,R')-COOH;-O-( )n-OH;OR',其中-R'为C1-C3烷基基团;-OH;-COOH;卤素,特别是-F,-Cl,-Br,-I,-CF3;-O ;-NO2;-CH=CH-COOH;-( )n-COOH;O-( )n-P03H2;O-(CF2)n-P03H2;O-( )n-S03H;O-( )n-CONHOH;O-( )n-四唑;O-( )n-羟基异噁唑-n=1至5,优选1-3;所述的次磷酸衍生物为对映异构体或对映体。
-
FUNCTIONALIZED PHENOLIC COMPOUNDS AND POLYMERS THEREFROM申请人:Bezwada Rao S.公开号:US20090170927A1公开(公告)日:2009-07-02The present invention relates to compounds of formula I, which are functionalized phenolic compounds, and polymers formed from the same. Ar—[O—(X) p —R′] q I Polymers formed from the functionalized phenolics are expected to have controllable degradation profiles, enabling them to release an active component over a desired time range. The polymers are also expected to be useful in a variety of medical applications.
-
Synthesis and biological activity of fibrate-based acyl- and alkyl-phenoxyacetic methyl esters and 1,2-dihydroquinolines作者:Abraham Pucheta、Aarón Mendieta、Damián A. Madrigal、Roberto I. Hernández-Benitez、Liseth Romero、Leticia Garduño-Siciliano、Catalina Rugerio-Escalona、María C. Cruz-López、Fabiola Jiménez、Alejandra Ramírez-Villalva、Aydeé Fuentes-Benites、Carlos González-Romero、Omar Gómez-García、Julio López、Miguel A. Vázquez、Blanca Rosales-Acosta、Lourdes Villa-Tanaca、Alfonso Sequeda-Juárez、Eva Ramón-Gallegos、Germán Chamorro-Cevallos、Francisco Delgado、Joaquín TamarizDOI:10.1007/s00044-019-02496-1日期:2020.3constituted by a fibrate-based structure was recently reported by our group, whose synthesis started from isovanillin derivatives. In the interest of evaluating the bioisosteric effect of the vanillin-based isomers on their antihyperlipidemic activity, the present study focuses on the synthesis of 5-acyl-1-phenoxyacetic methyl esters 5a–c and their saturated side-chain 5-alkyl-1-phenoxyacetates 6a–c. Their我们的小组最近报道了由基于纤维状结构的一系列高效降血脂药,其合成从异香草醛衍生物开始。为了评估基于香草醛的异构体对它们的降血脂活性的生物立体效应,本研究着重于5-酰基-1-苯氧基乙酸甲酯5a - c及其饱和侧链5-烷基-1的合成-苯氧乙酸酯6a – c。它们强大的体内作用与抑制HMG-CoA还原酶有关。由于1,2-二氢喹啉可抑制该酶,因此一系列此类杂环(9a – d)是通过我们有效的区域选择性,一步一步,无溶剂的方法制备的。除了在体内表现出降血脂活性外,某些化合物在体外还表现出抗真菌,抗氧化剂和细胞毒活性。通过对接模拟检查了四种化合物在HMGRh活性位点的结合模式,观察到与辛伐他汀靶向的大多数氨基酸发生相互作用。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
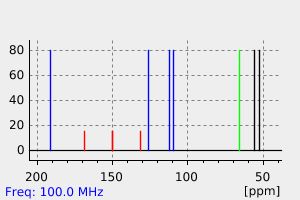
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息