(4Z)-4-十二碳烯 | 7206-27-1
中文名称
(4Z)-4-十二碳烯
中文别名
——
英文名称
(Z)-4-dodecene
英文别名
cis-4-dodecene;dodec-4c-ene;cis-4-Dodecen;Dodec-4c-en;(Z)-dodec-4-ene
CAS
7206-27-1
化学式
C12H24
mdl
——
分子量
168.323
InChiKey
PHCKFVVLVZFFLU-CLFYSBASSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-38.4°C (estimate)
-
沸点:207.3°C (estimate)
-
密度:0.7761 (estimate)
-
保留指数:1192;1191;1180
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:12
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (Z)-1-iodo-1-nonene 52886-17-6 C9H17I 252.138
反应信息
-
作为反应物:描述:(4Z)-4-十二碳烯 在 [OsIII(OH)(H2O)(N,N’-dimethyl-2,11-diaza-[3.3](2,6)pyridinophane)](PF6)2 、 双氧水 作用下, 以 水 、 正丁醇 为溶剂, 反应 5.0h, 以69%的产率得到参考文献:名称:带有大环配体的(III)和O(V)配合物:烯烃与过氧化氢的顺式-二羟基化反应的简单高效催化体系摘要:一个简单的协议,它使用[Os III(OH)(H 2 O)(L -N 4 Me 2)](PF 6)2(1 ; L- N 4 Me 2 = N,N'-二甲基-2,11 ‐diaza [3.3](2,6)pyridinophane)作为催化剂,H 2 O 2作为有效顺式的末端氧化剂介绍了烯烃的1,2-二羟基化反应。含有吸电子基团的未官能化(或脂肪族)烯烃和烯烃/苯乙烯被选择性氧化成相应的邻位二醇,收率良好至极佳(46–99%)。在催化反应中,烯烃:H 2 O 2的化学计量为1:1,因此氧化效率非常高。对于环己烯的二羟基化,可以将1的催化量降低至0.01 mol%,以实现5500的极高周转率。活性氧化剂被鉴定为Os V(O)(OH)种类(2)。通过氢过氧化物加合物形成的Os III(OOH)物种。活性氧化剂2 已成功分离并进行了晶体学表征。DOI:10.1002/asia.201300329
-
作为产物:描述:参考文献:名称:Synthesis of Regioselectively Deuterated Cyclopropanes摘要:A regioselective synthesis of deuterated aliphatic cyclopropanes has been developed to furnish labeled substrates for gas-phase ion-molecule reaction studies.DOI:10.1080/00397919608004635
文献信息
-
Le Bigot, Y.; Delmas, M.; Gaset, A., Synthetic Communications, 1982, vol. 12, # 2, p. 107 - 112作者:Le Bigot, Y.、Delmas, M.、Gaset, A.DOI:——日期:——
-
KATRITZKY, A. R.;EL-MOWAFY, AZZAHRA, M., J. ORG. CHEM., 1982, 47, N 18, 3506-3511作者:KATRITZKY, A. R.、EL-MOWAFY, AZZAHRA, M.DOI:——日期:——
-
LE, BIGOT YVES;DELMAS, MICHEL;GASET, ANTOINE, TETRAHEDRON, 44,(1988) N 4, 1057-1072作者:LE, BIGOT YVES、DELMAS, MICHEL、GASET, ANTOINEDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
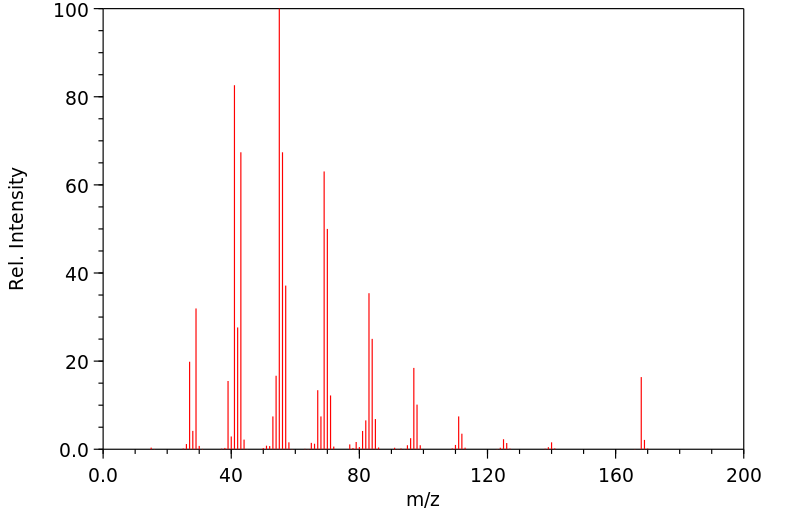
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-