庚二酸二乙酯 | 2050-20-6
中文名称
庚二酸二乙酯
中文别名
二乙基庚二酸醛;薄桃酸二乙酯
英文名称
diethyl pimelate
英文别名
diethyl heptanedioate
CAS
2050-20-6
化学式
C11H20O4
mdl
MFCD00009216
分子量
216.277
InChiKey
LKKOGZVQGQUVHF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-24°C
-
沸点:192-194 °C100 mm Hg(lit.)
-
密度:0.994 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
溶解度:1.97g/l微溶
-
蒸汽压力:0.00 mmHg
-
保留指数:1453
-
稳定性/保质期:
如果按照规定使用和存储,则不会分解,没有已知的危险反应,请避免接触氧化剂。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:15
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:0.818
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S23,S24/25
-
WGK Germany:3
-
海关编码:29171990
-
危险性防范说明:P233,P260,P261,P264,P271,P280,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
庚二酸二乙酯 修改号码:5
模块 1. 化学品
产品名称: Diethyl Pimelate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 庚二酸二乙酯
百分比: >97.0%(GC)
CAS编码: 2050-20-6
俗名: Diethyl Heptanedioate , Pimelic Acid Diethyl Ester , Heptanedioic Acid
Diethyl Ester
分子式: C11H20O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
庚二酸二乙酯 修改号码:5
模块 5. 消防措施
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 149 °C/2.4kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.99
溶解度:
[水] 无资料
[其他溶剂] 无资料
庚二酸二乙酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: UV9702000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
庚二酸二乙酯 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Diethyl Pimelate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 庚二酸二乙酯
百分比: >97.0%(GC)
CAS编码: 2050-20-6
俗名: Diethyl Heptanedioate , Pimelic Acid Diethyl Ester , Heptanedioic Acid
Diethyl Ester
分子式: C11H20O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
庚二酸二乙酯 修改号码:5
模块 5. 消防措施
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 149 °C/2.4kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.99
溶解度:
[水] 无资料
[其他溶剂] 无资料
庚二酸二乙酯 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: UV9702000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
庚二酸二乙酯 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-氯戊酸乙酯 ethyl 5-chloropentanoate 2323-81-1 C7H13ClO2 164.632 庚二酸 heptanedioic acid 111-16-0 C7H12O4 160.17 —— 4-hydroxyimino-heptanedioic acid diethyl ester 34999-74-1 C11H19NO5 245.276 4-碘-丁酸乙酯 ethyl 4-iodobutyrate 7425-53-8 C6H11IO2 242.057 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 庚二酸氢乙酯 7-ethoxy-7-oxoheptanoic acid 33018-91-6 C9H16O4 188.224 6-(氯甲酸基)己炔羧酸乙酯 ethyl 6-(chloroformyl)hexanoate 14794-32-2 C9H15ClO3 206.669 2,6-二溴庚二酸二乙酯 diethyl 2,6-dibromoheptanedioate 868-68-8 C11H18Br2O4 374.07 —— ethyl (8E,10Z)-7,12-dioxooctadeca-8,10-dienoate 122947-36-8 C20H32O4 336.472 —— ostopanic acid ethyl ester 122947-35-7 C20H32O4 336.472
反应信息
-
作为反应物:参考文献:名称:Willstaetter, Chemische Berichte, 1895, vol. 28, p. 660摘要:DOI:
-
作为产物:描述:参考文献:名称:US2014/256775摘要:公开号:
-
作为试剂:描述:辛二酸二乙酯 、 2-苯基氨基烟酸甲酯 在 sodium chloride 作用下, 以 庚二酸二乙酯 为溶剂, 生成 Ethyl 5-(4-hydroxy-2-oxo-1-phenyl-1H-[1,8]naphthyridin-3-yl)pentanoate 、 ethyl 6-(4-hydroxy-2-oxo-1-phenyl-1H-[1,8]naphthyridin-3-yl)-hexanoate参考文献:名称:Substituted hetero spiro pyridine derivatives as anti-allergy and摘要:替代异螺吡啶衍生物是抗过敏和抗炎药物。它们还可用于治疗消化性溃疡。其制备和使用方法已公开。公开号:US04632923A1
文献信息
-
Electron transfer reduction of unactivated esters using SmI2–H2O作者:Michal Szostak、Malcolm Spain、David J. ProcterDOI:10.1039/c1cc14014k日期:——The reduction of unactivated esters using samarium diiodide is reported for the first time. The optimised protocol allows for the reduction of primary, secondary and tertiary alkyl esters in excellent yields and is competitive with reductions mediated by metal hydrides and alkali metals.
-
Novel Myocyte Enhancer Factor 2 (MEF2) modulators申请人:STANDARD LLC公开号:US20190241504A1公开(公告)日:2019-08-08The present disclosure provides novel compounds capable of functioning as Myoctye Enhancer Factor 2 (MEF2) modulators, as well as compositions, pharmaceutical formulations, methods of synthesis and kits. Also provided are methods of treating a condition regulatable by MEF2 and/or MEF2 cofactors using the compounds, compositions, pharmaceutical formulations, and kits provided herein.本公开提供了能够作为肌肉细胞增强因子2(MEF2)调节剂发挥作用的新化合物,以及组合物、药物配方、合成方法和试剂盒。还提供了使用本文提供的化合物、组合物、药物配方和试剂盒治疗可通过MEF2和/或MEF2辅因子调节的疾病的方法。
-
Orthoamide, LXII [1]. – Mikrowellen-unterst ¨ utzte Formylierung schwach CH<sub>2</sub>-acider Verbindungen mit dem Bredereck-Simchen Reagenz – Umwandlung der Kondensationsprodukte in Heteroaromaten / Orthoamides, LXII [1]. – Microwave Assisted Formylation of Weak CH<sub>2</sub>-Acidic Compounds with the Bredereck-Simchen Reagent – Preparation of Heteroaromatic Compounds from the Condensation Products作者:Willi Kantlehner、Gerhard Simchen、Jochen Mezger、Edmont V. Stoyanov、Ralf Kreß、Wolfgang Frey、Björn SieversDOI:10.1515/znb-2005-0218日期:2005.2.1
The diformylation of the dinitriles 4 and diesters 7 with the Bredereck-Simchen reagent HC[N(CH3)2]2[OC(CH3)3] (1) under microwave irradiation give the bis-enamines 6 and 8 with dramatically reduced reaction times and improved yields compared to conventional heating. The condensation products formed can be easily converted to bis-pyrazole and bis-isoxazole derivatives 13 and 20, respectively. Methyl anthranilate reacts on prolonged heating with the orthoamide 21 to give ketene aminal 23 in low yield (8 %). Under microwave irradiation the same reagents lead to a mixture of 23 (14 %) and dihydropyrane 24 (28 %).
-
Phosphate tricyclic coumarin analogs as steroid sulfatase inhibitors: synthesis and biological activity作者:Witold Kozak、Mateusz Daśko、Maciej Masłyk、Jerzy S. Pieczykolan、Bartłomiej Gielniewski、Janusz Rachon、Sebastian DemkowiczDOI:10.1039/c4ra07135b日期:——biological evaluation of phosphate tricyclic coumarin derivatives as potential steroid sulfatase inhibitors. The described synthesis includes the straightforward preparation of 7-hydroxy-2,3-dihydro-1H-cyclopenta[c]chromen-4-one, 3-hydroxy-7,8,9,10-tetrahydro-6H-benzo[c]chromen-6-one and 3-hydroxy-8,9,10,11-tetrahydro-7H-cyclohepta[c]chromen-6-one modified with various phosphate moieties. The inhibitory在本工作中,我们报告了作为潜在的类固醇硫酸酯酶抑制剂的磷酸三环香豆素衍生物的合成和生物学评估的便捷方法。所描述的合成包括直接制备7-羟基-2,3-二氢-1 H-环戊基[ c ] chromen-4-one,3-羟基-7,8,9,10-四氢-6 H-苯并[ c ] chromen-6-one和3-羟基-8,9,10,11-四氢-7 H-环庚基[ c ] chromen-6-one,经各种磷酸盐部分修饰。测试了合成化合物对从人胎盘分离的STS以及MCF-7,MDA-MB-231和MDA-MB-435S癌细胞系的抑制作用。大多数新的STS抑制剂具有IC 50值介于21至159μM之间。在我们的研究过程中,对三种化合物9p,9r和9s的STS酶分析发现最大的抑制作用,IC 50值分别为36.4、37.8和21.5μM(IC 50值为1.0μM。 665-COUMATE作为参考)。化合物9r对MCF-7(雌激素受体阳性(ER
-
A New Coupling Reaction of Alkyl Iodides with Electron Deficient Alkenes Using Nickel Boride (cat.)−Borohydride Exchange Resin in Methanol作者:Tae Bo Sim、Jaesung Choi、Meyoung Ju Joung、Nung Min YoonDOI:10.1021/jo961751f日期:1997.4.1addition reaction of alkyl iodides with alpha,beta-unsaturated esters, nitriles, and ketones proceeds in moderate to excellent yields (50-95%) using Ni(OAc)(2) (0.05-0.2 equiv)-BER (3-5 equiv) in methanol in 1-9 h at room temperature or at 65 degrees C. Nickel boride on borohydride exchange resin (BER) is a good alternative reagent to tributyltin hydride for the coupling of alkyl iodides with the electron
表征谱图
-
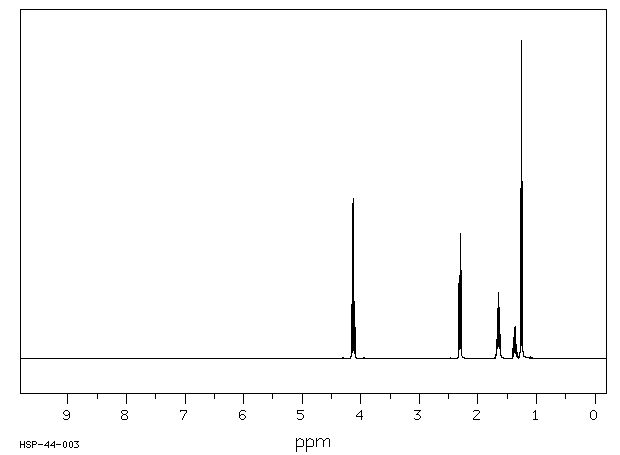
氢谱1HNMR
-
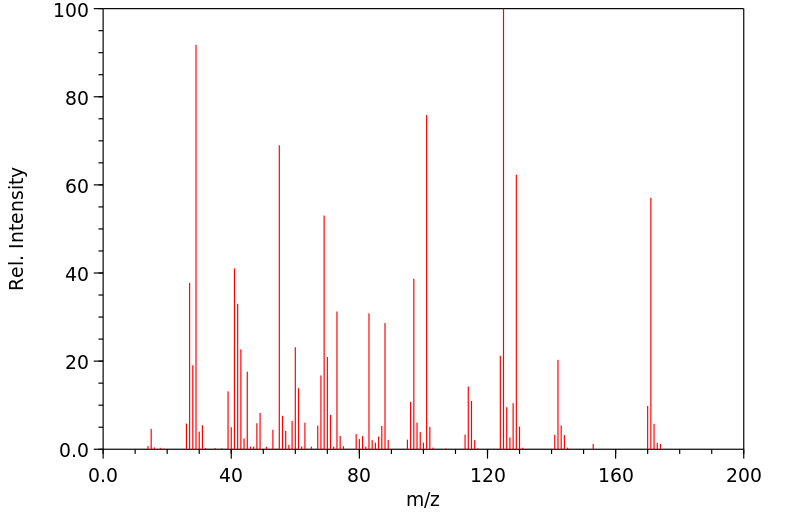
质谱MS
-
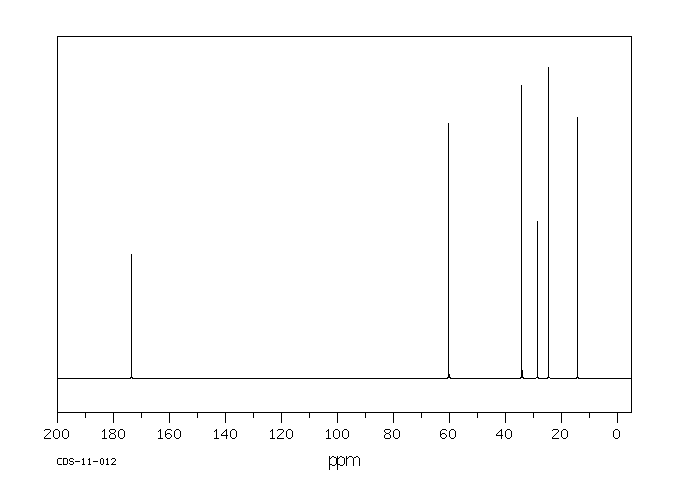
碳谱13CNMR
-
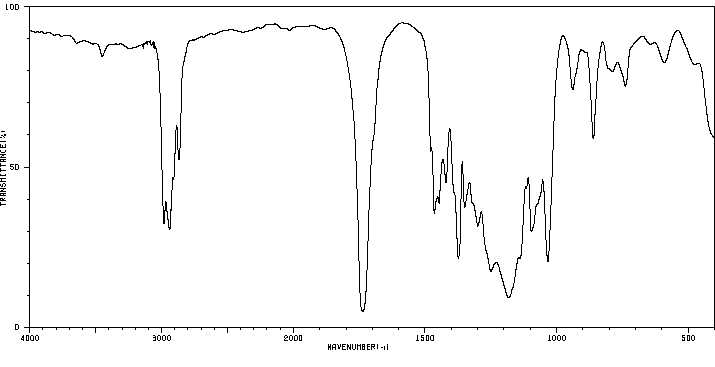
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯