异丙甲草胺 | 51218-45-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25°C
-
沸点:bp0.001 100°
-
密度:1.1200
-
闪点:2 °C
-
溶解度:可溶于氯仿(少许)、DMSO(少许)、甲醇(少许)
-
物理描述:Metolachlor is a tan to brown oily liquid with a slightly sweet odor. Slightly soluble in water and denser than water. Hence sinks in water. Soluble in most organic solvents. Used as a selective herbicide.
-
颜色/状态:Colorless liquid
-
蒸汽压力:3.14X10-5 mm Hg at 25 °C
-
亨利常数:9.00e-09 atm-m3/mole
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,也没有已知的危险反应。该物质对兔眼无刺激性,但对皮肤有轻度刺激。动物实验未发现其具有致畸、致癌或致突变的作用。
-
自燃温度:510 °F (USCG, 1999)
-
分解:When heated to decompostion it emits toxic fumes of /hydrogen chloride and nitrogen oxides/.
-
腐蚀性:Noncorrosive
-
折光率:Index of refraction: 1.5301 at 20 °C/D
-
碰撞截面:161.46 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:19
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.53
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
危险品标志:Xi
-
安全说明:S36/37
-
危险类别码:R43
-
WGK Germany:2
-
海关编码:2924299017
-
危险品运输编号:UN1648 3/PG 2
-
RTECS号:AN3430000
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境有良好的通风或排气设施。
SDS
制备方法与用途
毒性
- 大鼠急性经口LD50为2780mg/kg,急性经皮LD50>3170mg/kg ,急性吸入LC50>1750mg/m3 (4h)。对兔眼睛无刺激作用,皮肤有轻度刺激。大鼠90d饲喂试验无作用剂量为1000mg/kg,狗为500mg/kg。大鼠2年饲喂试验无作用剂量为1000mg/kg,小鼠为3000mg/kg。动物试验未见致畸、致癌、致突变作用。对鱼有毒,虹鳟鱼LC50为3.9mg/L, 鲇鱼LC50为4.9mg/L。对鸟低毒。对蜜蜂有胃毒,但无触杀毒性。
化学性质 纯品为无色液体,工业品为棕色油状液体。b.p.100℃/0.133Pa,蒸气压1.73×10-3Pa (20℃),相对密度1.12 (20)。能溶于甲醇、二氯乙烷等多种有机溶剂,在水中溶解度为530mg/L。不易光分解,贮存两年稳定,半衰期为25d。
用途 选择性苗前除草剂。禾本科杂草通过幼芽、幼根吸收药剂后,抑制蛋白质合成致死。适用于玉米、大豆、油菜、棉花、高粱、蔬菜等作物,防除马唐、稗草、牛筋草、狗尾草、千金子、画眉草等一年生禾本科杂草,对阔叶草防除效果较差,如阔叶草与禾本科杂草混生,可将两种药剂混合后使用。防除大豆、玉米田杂草,在播种后至出苗前用72%乳油,15~23mL/100m2对水喷雾土表。
用途 该品为芽前除草剂,主要用于防除禾本科杂草。产品属2-氯化乙酰替苯胺类除草剂,是细胞分裂抑制剂,用于土壤处理可防除稻田稗草、异型莎草、牛毛毡、鸭舌草、窄叶泽泻等。通常在插秧前3~5d使用。该品单施时对湿插水稻选择性较差,当与解草啶混用时效果更佳。制备方法一以2-乙基-6-甲基苯胺为原料,氨基上两个氢原子先与2-氯丙醇缩合,再与氯乙酰氯缩合生成中间体,最后与甲醇作用醚化生成异丙甲草胺。
生产方法二 2-乙基-6-甲基苯胺与2-溴-1-甲氧基丙烷于120℃、1.33×103Pa搅拌反应40h,得2-乙基-6-甲基-N-(1'-甲氧基-丙-2-基)苯胺,然后在三乙胺存在下,将上述中间体的苯溶液滴加到氯乙酰氯苯溶液中,室温搅拌反应2h。经水洗、干燥、蒸馏后得异丙甲草胺。
类别 农药
毒性分级 中毒
急性毒性
- 大鼠 LD50: 2200 毫克/ 公斤; 小鼠 LD50: 1150 毫克/ 公斤
刺激数据
- 皮肤-兔子:334 毫克 轻度;眼睛-兔子:100 毫克 轻度
可燃性危险特性 燃烧产生有毒氮氧化物和氯化物气体
储运特性 库房通风低温干燥; 与食品原料分开储运
灭火剂 干粉、泡沫、砂土
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丙草胺 2-chloro-2'-6'-diethyl-N-(propoxyethyl)acetanilide 51218-49-6 C17H26ClNO2 311.852 二甲草胺 2-chloro-N-(2,6-dimethylphenyl)-N-(2-methoxyethyl)-acetamide 50563-36-5 C13H18ClNO2 255.744 甲草胺 Alachlor 15972-60-8 C14H20ClNO2 269.771 乙草胺 Acetochlor 34256-82-1 C14H20ClNO2 269.771 丁草胺 N-butoxymethyl-2-chloro-N-(2,6-diethylphenyl)acetamide 23184-66-9 C17H26ClNO2 311.852 2-乙基-6-甲基-N-(1'-甲氧基-2'-丙基)苯胺 2-methyl-6-ethyl-N-(1-methyl-2-methoxyethyl)aniline 51219-00-2 C13H21NO 207.316 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-chloro-N-(2-ethyl-6-methylphenyl)-N-(2-hydroxy-1-methylethyl)acetamide 82535-98-6 C14H20ClNO2 269.771 异丙甲草胺去氯 2'-ethyl,6'-methyl-N-(methoxyprop-2-yl)acetanilide 126605-22-9 C15H23NO2 249.353 N-(2-乙基-6-甲基苯基)-2-羟基-N-(1-甲氧基丙烷-2-基)乙酰胺 2-hydroxy-N-(2-ethyl-6-methylphenyl)-N-(2-methoxy-1-methylethyl)acetamide 131068-72-9 C15H23NO3 265.353 —— 2-chloro-N-(2-ethyl-6-hydroxymethylphenyl)-N-(2-hydroxy-1-methylethyl)acetamide 96411-89-1 C14H20ClNO3 285.771 4-(2-乙基-6-甲基苯基)-5-甲基吗啉-3-酮 4-(2-ethyl-6-methylphenyl)-5-methyl-3-morpholinone 120375-14-6 C14H19NO2 233.31 —— 2-chloro-N-[2-(1-hydroxyethyl)-6-methylphenyl]-N-(2-hydroxy-1-methylethyl)acetamide 96394-95-5 C14H20ClNO3 285.771 2-[(2-乙基-6-甲基苯基)(1-甲氧基-2-丙基)氨基]-2-氧代乙烷磺酸 2-[(2-ethyl-6-methylphenyl)(2-methoxy-1-methylethyl)amino]-2-oxoethanesulfonic acid 171118-09-5 C15H23NO5S 329.417 —— 8-ethyl-3-hydroxy-N-(2-methoxy-1-methylethyl)-2-oxo-1,2,3,4-tetrahydroquinolinen 65513-63-5 C15H21NO3 263.337 N-(2-乙基-6-甲苯基)-2-羟基乙酰胺 Acetamide, N-(2-ethyl-6-methylphenyl)-2-hydroxy- 97055-05-5 C11H15NO2 193.24
反应信息
-
作为反应物:参考文献:名称:相邻酰胺基参与竞争性酸催化的醚键水解和分子内S N 2反应。2个摘要:使底物1经受酸水解,并且在竞争反应(平行途径)中获得两种反应产物(2,3)。通过使用平行的一级反应的表达式详细说明的实验数据显示,形成3(分子内环化)的速度比2(先前观察到的醚键的水解裂解)慢约三倍。两个反应似乎都由质子化的邻近酰胺基团辅助。DOI:10.1016/s0040-4020(97)00662-5
-
作为产物:描述:2-甲基-6-乙基苯胺 在 1-(n-butyl)-3-methylimidazolium tetrachloroaluminate 、 bis(1,5-cyclooctadiene)diiridium(I) dichloride 、 氢气 、 四丁基碘化铵 、 sodium carbonate 、 溶剂黄146 、 (2R,4R)-(+)-2,4-双(二苯基磷)戊烷 作用下, 以 甲苯 为溶剂, 80.0~85.0 ℃ 、4.5 MPa 条件下, 反应 22.0h, 生成 异丙甲草胺参考文献:名称:一种精异丙甲草胺的生产方法摘要:本发明属于有机合成技术领域,具体涉及一种精异丙甲草胺的生产方法。所述生产方法包括如下步骤:通过连续反应精馏制备亚胺,并使用酸性离子液体催化剂,收集中间体亚胺;接着采用高压环境氢化反应制备胺醚,所用铱盐催化剂活性高;最后通过酰化反应制备精异丙甲草胺,分离纯化得到产品精异丙甲草胺。本发明精异丙甲草胺生产高效连续,所得精异丙甲草胺收率高,纯度高,对映体过量值高。公开号:CN116478058A
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] 3-[(HYDRAZONO)METHYL]-N-(TETRAZOL-5-YL)-BENZAMIDE AND 3-[(HYDRAZONO)METHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE DERIVATIVES AS HERBICIDES<br/>[FR] DÉRIVÉS DE 3-[(HYDRAZONO))MÉTHYL]-N-(TÉTRAZOL-5-YL)-BENZAMIDE ET DE 3-[(HYDRAZONO)MÉTHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE UTILISÉS EN TANT QU'HERBICIDES申请人:SYNGENTA CROP PROTECTION AG公开号:WO2021013969A1公开(公告)日:2021-01-28The present invention related to compounds of Formula (I): or an agronomically acceptable salt thereof, wherein Q, R2, R3, R4, R5 and R6 are as described herein. The invention further relates to compositions comprising said compounds, to methods of controlling weeds using said compositions, and to the use of compounds of Formula (I) as a herbicide.本发明涉及以下式(I)的化合物或其农业上可接受的盐,其中Q、R2、R3、R4、R5和R6如本文所述。该发明还涉及包含所述化合物的组合物,使用这些组合物控制杂草的方法,以及将式(I)的化合物用作除草剂的用途。
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
[EN] HERBICIDALLY ACTIVE HETEROARYL-S?BSTIT?TED CYCLIC DIONES OR DERIVATIVES THEREOF<br/>[FR] DIONES CYCLIQUES SUBSTITUÉES PAR HÉTÉROARYLE À ACTIVITÉ HERBICIDE OU DÉRIVÉS DE CELLES-CI申请人:SYNGENTA LTD公开号:WO2011012862A1公开(公告)日:2011-02-03The invention relates to a compound of formula (I), which is suitable for use as a herbicide wherein G is hydrogen or an agriculturally acceptable metal, sulfonium, ammonium or latentiating group; Q is a unsubstituted or substituted C3-C8 saturated or mono-unsaturated heterocyclyl containing at least one heteroatom selected from O, N and S, or Q is heteroaryl or substituted heteroaryl; m is 1, 2 or 3; and Het is an optionally substituted monocyclic or bicyclic heteroaromatic ring; and wherein the compound is optionally an agronomically acceptable salt thereof.
-
TRIAZOLE ACC INHIBITORS AND USES THEREOF
表征谱图
-
氢谱1HNMR
-
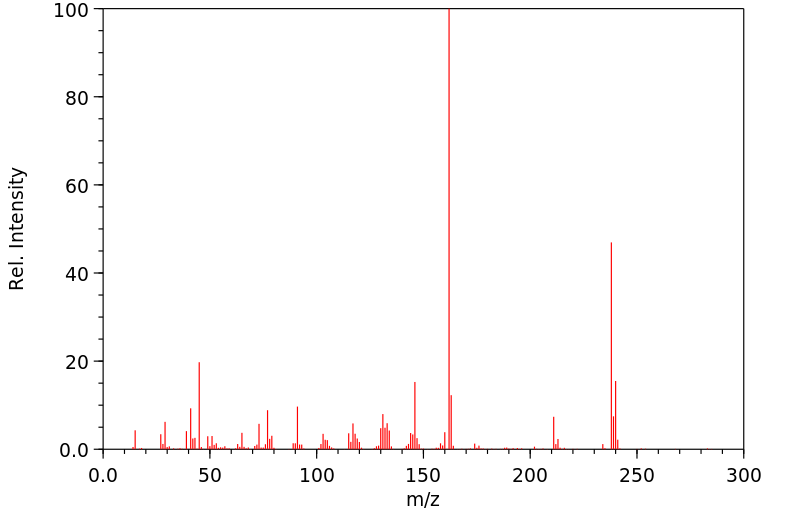
质谱MS
-
碳谱13CNMR
-
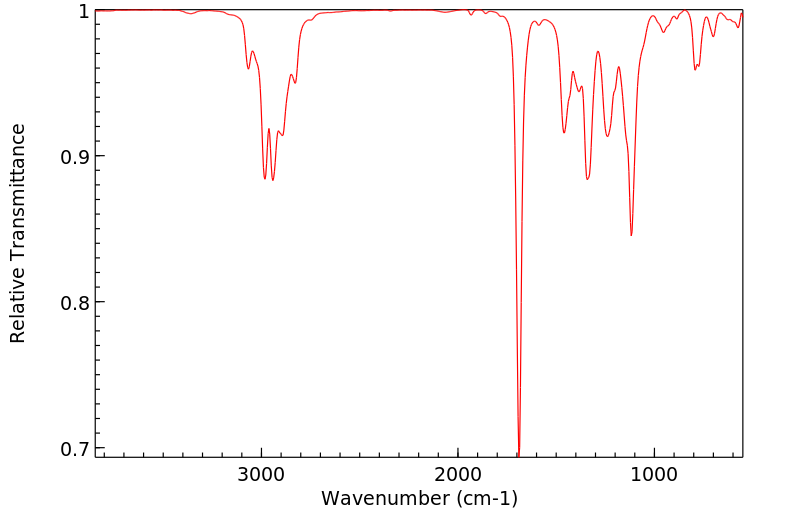
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息