乙草胺 | 34256-82-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:<0°C
-
沸点:bp0.4 torr 134°
-
密度:1.1
-
闪点:>68°C
-
溶解度:氯仿:微溶,DMSO:微溶,
-
LogP:3.030
-
颜色/状态:Clear, viscous liquid
-
气味:Aromatic odor
-
蒸汽压力:2.2X10-2 mPa /1.67X10-7 mm Hg/ at 20 °C
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
-
分解:When heated to decomposition it emits toxic vapors of /nitrogen oxides and hydrogen chloride/.
-
腐蚀性:Formulations are sightly corrosive to mild steel
-
折光率:Index of refraction: 1.5272 at 20 °C/D
-
碰撞截面:160.09 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
-
保留指数:1852
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:18
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S2,S36/37,S60,S61
-
危险类别码:R37/38,R50/53,R43,R20
-
WGK Germany:2
-
危险品运输编号:UN3082 9/PG 3
-
海关编码:3808931100
-
RTECS号:AB5457000
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Acetochlor
CAS-No. : 34256-82-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Inhalation (Category 4)
Specific target organ toxicity - single exposure (Category 3)
Skin irritation (Category 2)
Skin sensitization (Category 1)
Acute aquatic toxicity (Category 1)
Chronic aquatic toxicity (Category 1)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful by inhalation. Irritating to respiratory system and skin. May cause sensitization by skin contact.
Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H315 Causes skin irritation.
H317 May cause an allergic skin reaction.
H332 Harmful if inhaled.
H335 May cause respiratory irritation.
H410 Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P261 Avoid breathing vapours.
P273 Avoid release to the environment.
P280 Wear protective gloves.
P501 Dispose of contents/ container to an approved waste disposal plant.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R20 Harmful by inhalation.
R37/38 Irritating to respiratory system and skin.
R43 May cause sensitization by skin contact.
R50/53 Very toxic to aquatic organisms, may cause long-term adverse effects in
the aquatic environment.
S-phrase(s)
S36/37 Wear suitable protective clothing and gloves.
S60 This material and its container must be disposed of as hazardous waste.
S61 Avoid release to the environment. Refer to special instructions/ Safety
data sheets.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C14H20ClNO2
Molecular Weight : 269,77 g/mol
Component Concentration
Acetochlor
CAS-No. 34256-82-1 -
EC-No. 251-899-3
Index-No. 616-037-00-6
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas
Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation.
Evacuate personnel to safe areas.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
Methods and materials for containment and cleaning up
Soak up with inert absorbent material and dispose of as hazardous waste. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
Colour: light yellow
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 134 °C at 0,5 hPa
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,135 g/cm3
n) Water solubility 0,2 g/l at 20 °C
o) Partition coefficient: n- log Pow: 2,719
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
LD50 Oral - rat - 763 mg/kg
LD50 Dermal - rabbit - 4.166 mg/kg
Skin corrosion/irritation
Serious eye damage/eye irritation
Eyes - rabbit - Mild eye irritation - Draize Test
Respiratory or skin sensitization
May cause sensitization by skin contact.
Germ cell mutagenicity
Genotoxicity in vitro - Human - lymphocyte
Sister chromatid exchange
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Developmental Toxicity - rat - Oral
Effects on Embryo or Fetus: Fetotoxicity (except death, e.g., stunted fetus).
Specific target organ toxicity - single exposure
May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation Harmful if inhaled. Causes respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. Causes skin irritation.
Eyes Causes eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: AB5457000
Section 12. ECOLOGICAL INFORMATION
Toxicity
Toxicity to fish LC50 - Oncorhynchus mykiss (rainbow trout) - 0,38 mg/l - 96,0 h
Toxicity to daphnia and EC50 - Daphnia magna (Water flea) - 7,2 mg/l - 48 h
other aquatic
invertebrates
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
Very toxic to aquatic life.
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: 3082 IMDG: 3082 IATA: 3082
UN proper shipping name
ADR/RID: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID, N.O.S. (Acetochlor)
IMDG: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID, N.O.S. (Acetochlor)
IATA: Environmentally hazardous substance, liquid, n.o.s. (Acetochlor)
Transport hazard class(es)
ADR/RID: 9 IMDG: 9 IATA: 9
Packaging group
ADR/RID: III IMDG: III IATA: III
Environmental hazards
ADR/RID: yes IMDG Marine pollutant: yes IATA: yes
Special precautions for user
Further information
EHS-Mark required (ADR 2.2.9.1.10, IMDG code 2.10.3) for single packagings and combination
packagings containing inner packagings with Dangerous Goods > 5L for liquids or > 5kg for solids.
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
乙草胺是由孟山都和捷利康开发的一种除草剂,属于苯胺类。它作为elongase和香叶基香叶基焦磷酸(GGPP)环化酶的抑制剂发挥作用,从而阻断赤霉素途径。不过,这种化学物质具有较高的环境污染风险。
药效乙草胺的有效期约为40至70天,主要在土壤表层0~3厘米处保持活性。高温、高湿条件下或药后持续低温、高湿易产生药害,但一般情况下10~15天后可恢复正常。若播种后24~72小时内施药,则容易造成药害。杂草吸收的主要部位是芽,因此必须在杂草出土前施用。
毒性大鼠急性口服LD₅₀为2148毫克/千克(1160毫克/千克),兔急性经皮LD₅₀为794毫克/千克(4166毫克/千克,50%乳油)。虹鳟鱼LC₅₀为0.5毫克/升(96小时)。
化学性质乙草胺为浅棕色液体。沸点大于200℃,熔点大于0℃,蒸汽压133.3帕斯卡,相对密度在30℃时为1.11。其在25℃水中的溶解度为223毫克/升,不易挥发且对光稳定。
用途乙草胺是一种酰胺类选择性、芽前除草剂,适用于玉米、棉花、大豆、花生、油菜、马铃薯、甘蔗、芝麻、向日葵以及豆科、十字花科、茄科、菊科、伞形花科等多种蔬菜田及果园防除一年生禾本科杂草。一次施药可控制作物整个生育期无杂草危害,但对多年生杂草无效。
生产方法乙草胺的合成过程如下:2,6-甲基乙基苯胺与氯乙酸和三氯化磷反应生成2,6-甲基乙基氯代乙酰替苯胺;乙醇与聚甲醛在盐酸存在下反应,醚化后得到氯甲基乙基醚;最后,2-甲基-6-乙基氯代乙酰替苯胺与氯甲基乙基醚缩合,即得乙草胺。产品50%乳油为淡棕色液体,43%乳油为红棕色液体。原料消耗定额为:2,6-甲基乙基苯胺600千克/吨、氯乙酸480千克/吨、乙醇250千克/吨、聚甲醛150千克/吨、烧碱1100千克/吨。
合成步骤2,6-甲基乙基苯胺与氯乙酸和三氯化磷反应生成2,6-甲基乙基-2-氯代乙酰替苯胺。
乙醇与聚甲醛在盐酸存在下反应,生成氯甲基乙基醚。
2,6-甲基乙基-2-氯代乙酰替苯胺与氯甲基乙基醚缩合,得到乙草胺。
农药
毒性分级中毒
急性毒性口服 - 大鼠 LD₅₀: 763 毫克/千克
可燃性危险特性燃烧产生有毒氮氧化物和氯化物气体
储运特性库房应通风、低温干燥,并与食品原料分开储运。
灭火剂干粉、泡沫、砂土
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-(chloromethyl)-2'-methyl-6'-ethyl-2-chloroacetanilide 57415-63-1 C12H15Cl2NO 260.163 —— N-(2-methyl-6-ethyl)-phenyl-N-ethyloxymethyl-carbamoyl chloride 56917-59-0 C13H18ClNO2 255.744 2-乙基-6-甲基-2-氯乙酰苯胺 2-chloro-N-(2-ethyl-6-methylphenyl)acetamide 32428-71-0 C11H14ClNO 211.691 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 异丙甲草胺 2-chloro-6'-ethyl-N-(2-methoxy-1-methylethyl)acet-o-toluidide 51218-45-2 C15H22ClNO2 283.798 2-乙基-6-甲基-2-氯乙酰苯胺 2-chloro-N-(2-ethyl-6-methylphenyl)acetamide 32428-71-0 C11H14ClNO 211.691
反应信息
-
作为反应物:描述:参考文献:名称:Evaluation of the potential carcinogenicity and genetic toxicity to humans of the herbicide acetochlor摘要:本文介绍并讨论了除草剂乙草胺的全面毒理学研究。尽管在许多毒性测试中它给出了负面的反应概况,但还是有一些发现促使进一步调查。首先,尽管在Salmonella试验中不具有突变原性,但在体外处理的哺乳动物细胞中,乙草胺具有染色体破裂原性。然而,在四种啮齿动物细胞遗传学试验(骨髓和生殖细胞)中,在体内未表达出这种染色体破裂潜力。其次,尽管在急性最大耐受剂量(MTD)下进行大鼠肝脏UDS试验时,乙草胺没有反应,但单次超过MTD剂量(2000mg/kg)的灌胃给药却产生了微弱的阳性试验反应。这个剂量水平(2000mg/kg)对肝脏是坏死性的,可使肝脏谷胱甘肽水平降低到80%左右,改变乙草胺的代谢,并伴有高达33%的致死率。相比之下,参考肝脏基因毒素如DMN、DMH和2AAF,在没有这种影响的情况下,在低至约400倍的剂量水平下引起UDS。最后,当乙草胺以最大耐受剂量(MTD)在大鼠体内给药两年时,会诱导大鼠鼻粘膜息肉状腺瘤。这些肿瘤不会危及生命,不会转移,且在饮用含MTD乙草胺的饮食1或18周后,大鼠鼻细胞中没有诱导DNA损伤(彗星试验)。 为了探究这些高剂量毒性的作用机制,对乙草胺和一系列结构类似物进行了一系列化学和遗传毒性研究。这些研究揭示了氯乙酰亚基结构在体外是染色体破裂的物质。虽然相对惰性,但这种取代基更倾向于与巯基结构反应,最明显的是与谷胱甘肽(GSH)反应。对于两种带有氯乙酰基团的相关化合物,体外的化学反应性和染色体破裂性也被观察到,这两种化合物在美国NTP报告的研究中被定义为非致癌物质。这些观察结果表明,在体外,乙草胺的染色体破裂性源也是它在大鼠体内通过与GSH反应快速解毒的源。描述了乙草胺的代谢研究,发现在大鼠中形成了一系列与GSH相关的胆汁代谢物,而在小鼠中则没有产生。乙草胺在大鼠中的代谢会随着剂量水平的增加而改变,可能是因为肝脏GSH的耗竭。 很可能是大鼠特有的代谢产物导致了观察到的大鼠鼻肿瘤,只有在高剂量水平下才会出现。急性或亚慢性接触乙草胺的大鼠鼻上皮细胞没有遗传毒性,有利于采用非遗传性机制诱导这些腺瘤。观察到与时间和剂量相关的S期细胞在鼻上皮中增加,与这个结论是一致的。 尽管早期对啮齿动物进行了致死性灌胃给药和一些慢性研究中使用了超过MTD的饮食浓度,但可用的MTD数据表明,乙草胺对人类不构成遗传或致癌危害。DOI:10.1177/096032719601500902
-
作为产物:描述:2-chloro-N-(ethoxymethyl)-N-(2-ethyl-4-iodo-6-methylphenyl)acetamide 在 palladium on activated charcoal 氢气 、 三乙胺 作用下, 以 乙醇 为溶剂, 反应 3.0h, 以14 mg的产率得到乙草胺参考文献:名称:Radiosynthesis of a chloroacetanilide herbicide ([phenyl-4-3H] acetochlor) and a dichloroacetamide safener for herbicides ([2, 2-dimethyl-3H]R-29148)摘要:2-氯-N-(乙氧基甲基)-N-(2-乙基-6-甲基苯基)乙酰胺(氯乙酰苯胺除草剂乙草胺)和3-(二氯乙酰基)-2,2,5-三甲基-1,3-恶唑烷(二氯乙酰胺安全剂R-29148)需要高比活度,用于研究其代谢和作用方式。在碳钯和三乙胺的作用下,碘乙草胺在乙醇中与氚气发生还原脱卤反应,得到[苯基-4-3H]乙草胺,产率为71%,浓度为22 Ci/mmol。在五烷中,用等量的NaOH处理丙酮和1-氨基-2-丙醇,得到[2,2-二甲基-3H]R-29148,浓度为15 Ci/mmol,然后在二氯乙酰氯的作用下,得到[2,2-二甲基-3H]R-29148。DOI:10.1002/jlcr.2580360207
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] 3-[(HYDRAZONO)METHYL]-N-(TETRAZOL-5-YL)-BENZAMIDE AND 3-[(HYDRAZONO)METHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE DERIVATIVES AS HERBICIDES<br/>[FR] DÉRIVÉS DE 3-[(HYDRAZONO))MÉTHYL]-N-(TÉTRAZOL-5-YL)-BENZAMIDE ET DE 3-[(HYDRAZONO)MÉTHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE UTILISÉS EN TANT QU'HERBICIDES申请人:SYNGENTA CROP PROTECTION AG公开号:WO2021013969A1公开(公告)日:2021-01-28The present invention related to compounds of Formula (I): or an agronomically acceptable salt thereof, wherein Q, R2, R3, R4, R5 and R6 are as described herein. The invention further relates to compositions comprising said compounds, to methods of controlling weeds using said compositions, and to the use of compounds of Formula (I) as a herbicide.本发明涉及以下式(I)的化合物或其农业上可接受的盐,其中Q、R2、R3、R4、R5和R6如本文所述。该发明还涉及包含所述化合物的组合物,使用这些组合物控制杂草的方法,以及将式(I)的化合物用作除草剂的用途。
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
[EN] HERBICIDALLY ACTIVE HETEROARYL-S?BSTIT?TED CYCLIC DIONES OR DERIVATIVES THEREOF<br/>[FR] DIONES CYCLIQUES SUBSTITUÉES PAR HÉTÉROARYLE À ACTIVITÉ HERBICIDE OU DÉRIVÉS DE CELLES-CI申请人:SYNGENTA LTD公开号:WO2011012862A1公开(公告)日:2011-02-03The invention relates to a compound of formula (I), which is suitable for use as a herbicide wherein G is hydrogen or an agriculturally acceptable metal, sulfonium, ammonium or latentiating group; Q is a unsubstituted or substituted C3-C8 saturated or mono-unsaturated heterocyclyl containing at least one heteroatom selected from O, N and S, or Q is heteroaryl or substituted heteroaryl; m is 1, 2 or 3; and Het is an optionally substituted monocyclic or bicyclic heteroaromatic ring; and wherein the compound is optionally an agronomically acceptable salt thereof.
-
TRIAZOLE ACC INHIBITORS AND USES THEREOF
表征谱图
-
氢谱1HNMR
-
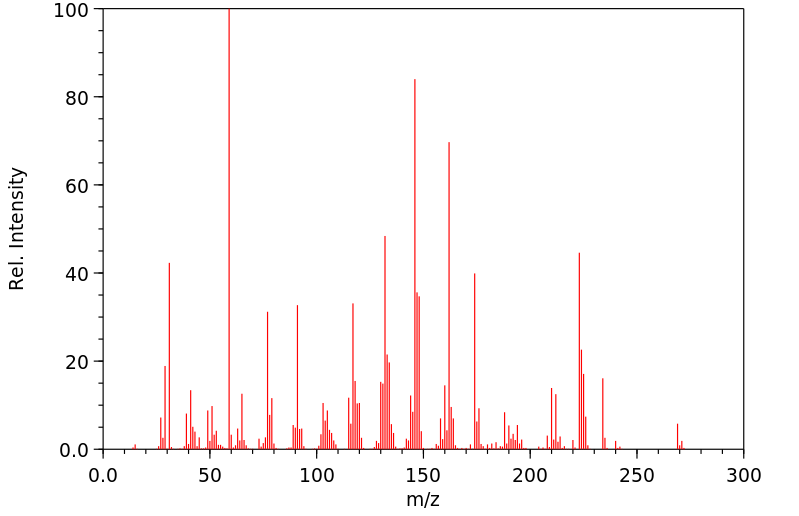
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息