1,1,1,2-四氯-2,2-二氟乙烷 | 76-11-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:39-44 °C
-
沸点:91-91 °C
-
密度:1.64
-
闪点:90-92°C
-
溶解度:溶于酒精、乙醚和氯仿(Weast,1986)
-
暴露限值:NIOSH REL: TWA 500 ppm (4,170 mg/m3), IDLH 2,000 ppm; OSHA PEL: TWA 500 ppm; ACGIH TLV: TWA 500 ppm (adopted).
-
物理描述:1,1,1,2-tetrachloro-2,2-difluoroethane is a colorless solid with a slight, ether-like odor. mp: 40.6°C; bp: 91.5°C.
-
颜色/状态:Colorless liquid or solid
-
气味:Slight ether odor
-
蒸汽密度:7.0 (Air = 1)
-
蒸汽压力:60 mm Hg at 25 °C
-
分解:Decomposes on contact with hot surfaces or flames. This produces toxic fumes including hydrogen chloride, hydrogen fluoride and phosgene.
-
粘度:2.1583X10-3 Pa.s at 313.75 K
-
腐蚀性:Special precautions: 1,1,1,2-tetrachloro-2,2-difluoroethane will attack some forms of plastics, rubber, and coatings.
-
汽化热:3.4838X10+7 J/mol at 313.75 K
-
表面张力:2.7913X10-2 N/m at 313.75 K
-
相对蒸发率:Greater than 1 (Butyl acetate = 1)
-
保留指数:702;702;785;785
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 500 ppm (4170 mg/m3)
-
立即威胁生命和健康浓度:2,000 ppm
-
危险品标志:Xi,N
-
安全说明:S26,S37/39,S59
-
危险类别码:R36/37/38,R59
-
海关编码:29034520
SDS
制备方法与用途
化学性质:
- 熔点:38-40℃
- 沸点:91℃
- 相对密度(20/4℃):1.65
用途: 用于合成麻醉药甲氧氟烷的中间体。
生产方法: 在搅拌下,将无水三氯化铝迅速加入已熔化的氟里昂112中,保温搅拌2-3小时后升温至60-70℃,保温过滤。滤液进行分馏,收集91-92℃的馏分以得到2,2-二氟四氯乙烷,收率为70%。
类别: 有毒物质
毒性分级: 低毒
急性毒性:
- 口服 - 大鼠 LD50: >8000 毫克/公斤
- 吸入 - 大鼠 LCLo: 20,000 ppm/30 分钟
储运特性: 需库房低温、通风且干燥,并密封保存。
职业标准: 时间加权平均容许浓度(TWA)为4170 毫克/立方米。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,2-三氯-1,1-二氟乙烷 1,1,2-trichloro-2,2-difluoroethane 354-21-2 C2HCl3F2 169.386 1,1,1-三氯-2,2-二氟乙烷 1,1,1-trichloro-2,2-difluoroethane 354-12-1 C2HCl3F2 169.386 1,1-二氟-1,2-二氯乙烷 1,2-dichloro-1,1-difluoroethane 1649-08-7 C2H2Cl2F2 134.941 1,1,2-三氯三氟乙烷(CFC-113) 1,1,2-Trichloro-1,2,2-trifluoroethane 76-13-1 C2Cl3F3 187.376 三氯三氟乙烷 1,1,1-Trichloro-2,2,2-trifluoroethane 354-58-5 C2Cl3F3 187.376 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 氟五氯乙烷 freon-111 354-56-3 C2Cl5F 220.285 1,1,2-三氯三氟乙烷(CFC-113) 1,1,2-Trichloro-1,2,2-trifluoroethane 76-13-1 C2Cl3F3 187.376 三氯三氟乙烷 1,1,1-Trichloro-2,2,2-trifluoroethane 354-58-5 C2Cl3F3 187.376
反应信息
-
作为反应物:描述:1,1,1,2-四氯-2,2-二氟乙烷 在 ammonium peroxydisulfate 、 sodium formate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 4.0h, 以66.5%的产率得到二氟氯乙酸参考文献:名称:全卤代烃的反应。第九部分 用氧化还原系统将全(聚)氟代烷基卤化物转化为相应的羧酸摘要:描述了用氧化还原体系-(NH 4)2 S 2 O 8 / HCO 2 Na将全(聚)氟代烷基卤化物转化为相应的羧酸。该反应为在温和条件下合成各种全(聚)氟羧酸提供了一种方便的方法。DOI:10.1016/s0022-1139(00)80387-5
-
作为产物:参考文献:名称:Henne; Ladd, Journal of the American Chemical Society, 1936, vol. 58, p. 403摘要:DOI:
-
作为试剂:描述:亚甲基三苯基膦烷 在 1,1,1,2-四氯-2,2-二氟乙烷 作用下, 以 乙醚 为溶剂, 反应 0.5h, 以15%的产率得到Dichlormethyl-triphenyl-phosphonium-bromid参考文献:名称:磷鎓叶立德与全卤代烷烃的新型反应:磷鎓叶立德的嗜盐攻击的第一个实例以及制备α-卤代烷基phosph盐的简便途径摘要:鏻叶立德博士3 PCHR(R = H,CH 3,C 2 H ^ 5,NC 3 ħ 7,NC 5 H ^ 11)容易反应与perhalofluoroalkanes,得到区域专一性α-haloalkylphosphonium盐博士3 P + -CHXRÿ - (X = I,Br,Cl),收率高。这些反应揭示了yl的一种新型反应性,即对CX键的嗜盐攻击,对于通过维蒂希反应进行取代卤代烯烃的区域特异性合成可能有用。DOI:10.1016/s0040-4039(01)91362-0
文献信息
-
Haloalkoxy anilide derivatives of 2-4(-heterocyclic oxyphenoxy)alkanoic申请人:DowElanco公开号:US05250690A1公开(公告)日:1993-10-05The present invention is directed to novel substituted aniline compounds, the optically active isomers of said compounds, compositions containing said compounds, and the use of these compounds in the selective kill and control of grassy weeds in the presence of valuable crop plants, especially corn plants.本发明涉及新型的取代苯胺化合物、所述化合物的光学活性对映异构体、含有所述化合物的组合物,以及这些化合物在选择性杀除和控制禾本科杂草特别是在玉米植物中的有价值作物植物的应用。
-
Process for preparing polyfluorochlorocarbonyl chlorides and申请人:Solvay Fluor und Derivate GmbH公开号:US05569782A1公开(公告)日:1996-10-29A process for preparing polyfluorochloro- and perfluorocarbonyl chlorides, for example for preparing perfluoropropionyl chloride, chlorodifluoroacetyl chloride or trifluoroacetyl chloride, in which starting materials are employed which have a CHCl.sub.2 group which is converted to a C(O)Cl group by photochemical oxidation with oxygen in the presence of added elemental chlorine and under exposure to activating irradiation by light having a wavelength .lambda..gtoreq.290 nm. The procedure is preferably unpressurized. Outstanding conversions with high selectivity are achieved using doped Hg light sources.
-
Process for the production of fluorinated organic compounds and fluorinating agents申请人:——公开号:US20030176747A1公开(公告)日:2003-09-18A process for the production of a fluorinated organic compound, characterized by fluorinating an organic compound having a hydrogen atoms using IF 5 ; and a novel fluorination process for fluorinating an organic compound having a hydrogen atoms by using a fluorinating agent containing IF 5 and at least one member selected from the group consisting of acids, bases, salts and additives.
-
gem-Difluorovinyl Derivatives as Insecticides and Acaricides作者:Thomas Pitterna、Manfred Böger、Peter MaienfischDOI:10.2533/000942904777678163日期:——
The insecticidal lead 1,1-difluorododec-1-ene was optimised. This compound has attractive insecticidal activity against tobacco budworm (Heliothis virescens), banded cucumber beetle (Diabrotica balteata), pea aphid (Aphis cracciovora), brown planthopper (Nilaparvata lugens), and green rice leafhopper (Nephotettix cincticeps). Among different pharmacophore analogues, only 1,1-dichlorododec-1-ene and 1,1-difluoro-2-iodododec-1-ene showed weak insecticidal activity, whereas similar compounds such as 1-chloro-1-fluorododec-1-ene, 1-fluorododec-1-ene, and 1,1-difluoro-2-bromododec-1-ene were inactive. Only bridge analogues with even-numbered carbon chains were active, for example 1,1-difluorodec-1-ene and 1,1-difluorotetradec-1-ene. Odd-numbered analogues such as 1,1-difluoronon-1-ene, 1,1-difluoroundec-1-ene, 1,1-difluorotridec-1-ene, and 1,1-difluoro-pentadec-1-ene showed no activity. Modification of the tail group led to the analogues 12,12-difluorododec-11-enoic acid and its methyl ester, 12,12-difluorododec-11-en-1-ol, 1,1-difluoro-12-methoxydodec-1-ene, and 12,12-difluorododec-11-enylamine, all of which showed insecticidal activity. 12,12-difluorododec-11-enoic acid methyl ester, 12,12-difluorododec-11-enoic acid, and 12,12-difluorododec-11-en-1-ol were also active against spider mites (Tetranychus ssp). Thus, in a first optimisation cycle, broad activity against insect pests and mites was discovered. Two requirements, the gem-difluorovinyl pharmacophore and an even-numbered carbon chain, were found to be essential for activity. This latter requirement is in line with the proposed mode of action, which involves inhibition of the ? -oxidation of fatty acids in insect mitochondria. In a second optimisation cycle, it was found that 6,6-difluorohex-5-enoic acid and its derivatives, such as acids, amides, and hydrazides, possess even superior properties as insecticides and acaricides. This led to the discovery of 6,6-difluorohex-5-enoic acid 2-[4-(4-trifluoromethylbenzyloxy)-phenoxy]-ethyl ester (CGA 304'111). This compound showed excellent performance in field trials against a wide range of pests, as well as a more favourable toxicological profile than earlier derivatives. For a largescale synthesis of CGA 304'111, five different synthetic routes for 6,6-difluorohex-5-enoic acid were developed. The best route involved radical addition of CBrClF2 to pent-4-enoic acid. Removal of bromine by hydrogenation, elimination of chloride and hydrolysis of the ester concluded this most efficient sequence. Thus, a practical synthesis for CGA 304'111 was identified, which allowed the preparation of samples on a several 100 g scale.
1,1-二氟十二烯是一种杀虫剂铅化合物,具有对烟草夜蛾、条纹黄瓜甲(Diabrotica balteata)、豌豆蚜虫(Aphis cracciovora)、褐飞虱(Nilaparvata lugens)和绿稻叶蝉(Nephotettix cincticeps)等昆虫的吸引力杀虫活性。在不同的药效团类似物中,只有1,1-二氯十二烯和1,1-二氟-2-碘十二烯表现出微弱的杀虫活性,而类似化合物如1-氯-1-氟十二烯、1-氟十二烯和1,1-二氟-2-溴十二烯则无活性。只有具有偶数碳链的桥接类似物才具有活性,例如1,1-二氟十烯和1,1-二氟十四烯。奇数碳链的类似物如1,1-二氟壬-1-烯、1,1-二氟十一-烯、1,1-二氟十三-烯和1,1-二氟十五-烯均没有活性。修改尾基导致类似物12,12-二氟十二-11-烯酸及其甲酯、12,12-二氟十二-11-烯-1-醇、1,1-二氟-12-甲氧基十二烯和12,12-二氟十二-11-烯基胺,均表现出杀虫活性。12,12-二氟十二-11-烯酸甲酯、12,12-二氟十二-11-烯酸和12,12-二氟十二-11-烯-1-醇也对蜘蛛螨(Tetranychus ssp)具有活性。因此,在第一个优化周期中,发现了对昆虫害虫和螨类广泛活性。两个要求,即gem-二氟乙烯药效团和偶数碳链,被发现是活性所必需的。后一个要求符合所提出的作用模式,涉及对昆虫线粒体中脂肪酸的β-氧化的抑制。在第二个优化周期中,发现6,6-二氟己-5-烯酸及其衍生物,如酸、酰胺和肼,具有更优越的杀虫剂和杀螨剂性能。这导致了6,6-二氟己-5-烯酸2-[4-(4-三氟甲基苄氧基)-苯氧基]-乙酯(CGA 304'111)的发现。这种化合物在田间试验中表现出对多种害虫的优异性能,且毒理学特性比早期衍生物更有利。为了大规模合成CGA 304'111,开发了六种不同的6,6-二氟己-5-烯酸合成路线。最佳路线涉及对戊-4-烯酸进行自由基加成反应。通过氢化去除溴,消除氯并水解酯结束了这个最有效的序列。因此,确定了CGA 304'111的实用合成方法,可以在数百克的规模上制备样品。 -
High surface area chromium(III) fluoride – Preparation and some properties作者:Gašper Tavčar、Tomaž SkapinDOI:10.1016/j.jfluchem.2019.04.019日期:2019.6Reaction of hydrated hydrazinium fluorochromate(III), [N2H6][CrF5]·H2O, with fluorine (F2) in anhydrous hydrogen fluoride (aHF) medium at room temperature yields completely amorphous CrF3-based materials with exceptionally high specific surface areas of 180–420 m2 g−1 (HS-CrF3). The stepwise reaction starts with the oxidative decomposition of the cationic part of the precursor with F2 that gives a在室温下,无水氟化氢(aHF)介质中水合氟铬酸铬(III)[N 2 H 6 ] [CrF 5 ]·H 2 O与氟(F 2)的反应生成完全无定形的CrF 3基材料比表面积极高,为180-420 m 2 g -1(HS-CrF 3)。逐步反应从前体的阳离子部分与F 2的氧化分解开始,得到具有低表面积的CrF 3中间体。在接下来的步骤中,部分Cr 3+被氧化为Cr > 3+,并且在存在残留的H 2 O / [H 3 O] +物质的情况下,会形成Cr > 3+的氟化物。挥发性铬化合物(主要是CrO 2 F 2)的形成显然是HS-CrF 3形成的关键步骤。从最终产品中除去这些成分会降低氧含量,并产生微孔。HS-CrF 3材料完全是非晶态的,其总体组成接近化学计量的CrF 3。最终产品中的少量Cr > 3+和氧很可能源自保留的非挥发性CrOF 3。HS-CrF 3材料是路易斯酸,并且对氯氟烃(CFC
表征谱图
-
氢谱1HNMR
-
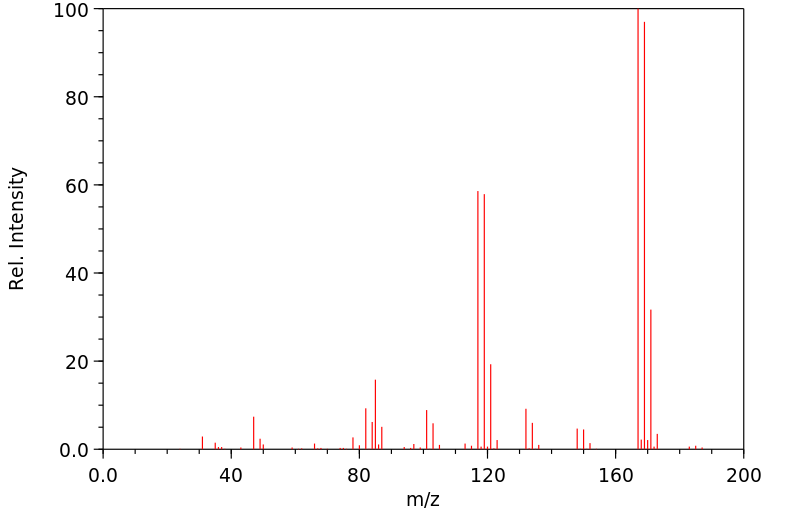
质谱MS
-
碳谱13CNMR
-
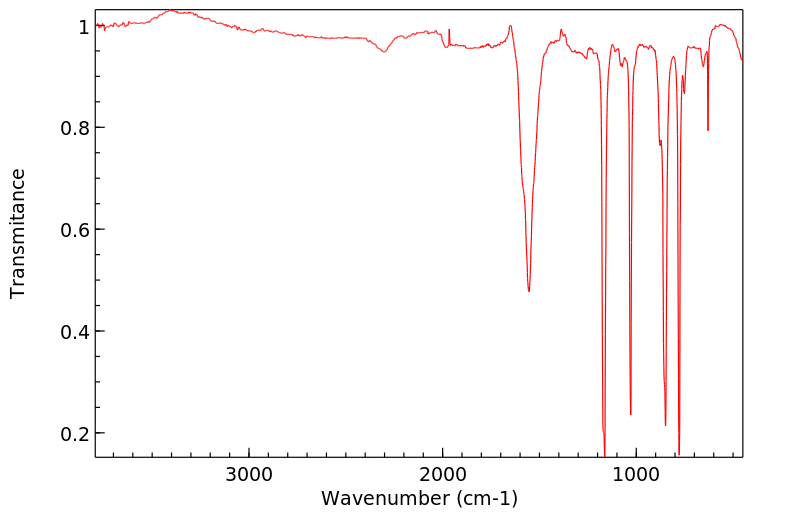
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息