1,2-二溴癸烷 | 28467-71-2
中文名称
1,2-二溴癸烷
中文别名
——
英文名称
1,2-dibromodecane
英文别名
——
CAS
28467-71-2
化学式
C10H20Br2
mdl
——
分子量
300.077
InChiKey
XBRBOTTWTQOCJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:250.72°C
-
密度:1.4630 (estimate)
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:12
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903399090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:邻位二溴化物的催化脱溴摘要:使用硼氢化钠和催化量的双(2-噻吩基)二碲化物将许多邻位二溴化物脱溴。DOI:10.1016/s0040-4039(00)87681-9
-
作为产物:参考文献:名称:vic-Dibromides与Diorganotellurides的脱溴。1.立体选择性,相对比率和机理含义。摘要:描述了用二芳基碲化物1-4和二正己基碲化物(9)对vic-二溴化物进行脱溴。提供了脱溴机理的解释,这解释了几个关键的实验观察结果:(1)该反应具有立体立体选择性,其中赤-二溴化物提供反式烯烃,而苏-二溴化物提供顺式烯烃,(2)该反应可进一步促进反应。富含电子的二有机碲化物,(3)在极性更大的溶剂中加速反应,(4)通过向带有溴取代基的碳中添加稳定碳阳离子的取代基来加速反应,(5)赤型二溴化物非常多比苏式二溴化物更具反应性。有人提出,维他命二溴化物形成溴离子的速度很慢,而且速度决定。形成溴离子后,快速清除“DOI:10.1021/jo9713363
-
作为试剂:参考文献:名称:通过镁和1,2-二溴乙烷还原Cp 2 TiCl 2和Cp 2 ZrCl 2生成的低价钛和锆试剂的新型反应摘要:在0°C下,用格氏(Grignard)级镁和1,2-二溴乙烷还原Cp 2 MCl 2(M = Ti或Zr),得到相应的茂金属-乙烯配合物和氢化物,如与二苯基-乙炔制得1,2-二苯基-(E)-1-丁烯。还描述了Cp 2 ZrCl 2 / 1,2-二溴苯/ Mg体系在THF中与二苯乙炔和降冰片烯的反应。DOI:10.1016/0022-328x(88)83026-2
文献信息
-
Mild and Efficient Vicinal Dibromination of Olefins Mediated by Aqueous Ammonium Fluoride作者:Tony Shing、Ying-Yeung Yeung、Wing NgDOI:10.1055/s-0036-1588506日期:2018.3A mild and efficient vicinal dibromination of olefins has been developed by using saturated aqueous ammonium fluoride solution as the promoter. Inexpensive and commercially available N -bromosuccinimide (NBS) was used as the brominating reagent. The corresponding vicinal dibromoalkanes could be obtained in good to excellent yields.
-
A Photoirradiative Phase-Vanishing Method: Efficient Generation of HBr from Alkanes and Molecular Bromine and Its Use for Subsequent Radical Addition to Terminal Alkenes作者:Hiroshi Matsubara、Ilhyong Ryu、Masaaki Tsukida、Daisuke Ishihara、Kenji KuniyoshiDOI:10.1055/s-0030-1258482日期:2010.8phase-vanishing (PV) system comprised of an alkane, perfluorohexanes, and bromine was successfully combined by photoirradiation to efficiently generate hydrogen bromide, which underwent radical addition with 1-alkenes in the hydrocarbon layer to afford terminal bromides in high yields.
-
Vanadium-catalyzed oxidative bromination promoted by Brønsted acid or Lewis acid作者:Kotaro Kikushima、Toshiyuki Moriuchi、Toshikazu HiraoDOI:10.1016/j.tet.2010.06.042日期:2010.8The oxidative bromination of arenes was induced by a vanadium catalyst in the presence of a bromide salt and a Brønsted acid or a Lewis acid under molecular oxygen, which provides an eco-friendly bromination method as compared with a conventional bromination one with bromine. This catalytic reaction could be applied to the bromination of alkenes and alkynes to give the corresponding vic-bromides. Use
-
Efficient bromination of olefins, alkynes, and ketones with dimethyl sulfoxide and hydrobromic acid作者:Song Song、Xinwei Li、Xiang Sun、Yizhi Yuan、Ning JiaoDOI:10.1039/c5gc00528k日期:——The manuscript describes a novel and efficient oxidative bromination of olefins, alkynes, and ketones with HBr as the brominating reagent and DMSO as the mild oxidant. This chemistry provides an...
-
A convenient synthesis of 1- and 2-alkynes作者:J. Klein、E. GurfinkelDOI:10.1016/s0040-4020(01)92790-5日期:1970.1Terminal olefins are transformed by bromination and subsequent dehydrobromination at room temperature with a solution of sodamide or sodium hydride in DMSO to pure terminal acetylenes. trans Dehydrobromination of vinylic bromides proceeds faster than the cis. Conditions were found, where the 1-alkynes are converted into 2-alkynes.
表征谱图
-
氢谱1HNMR
-
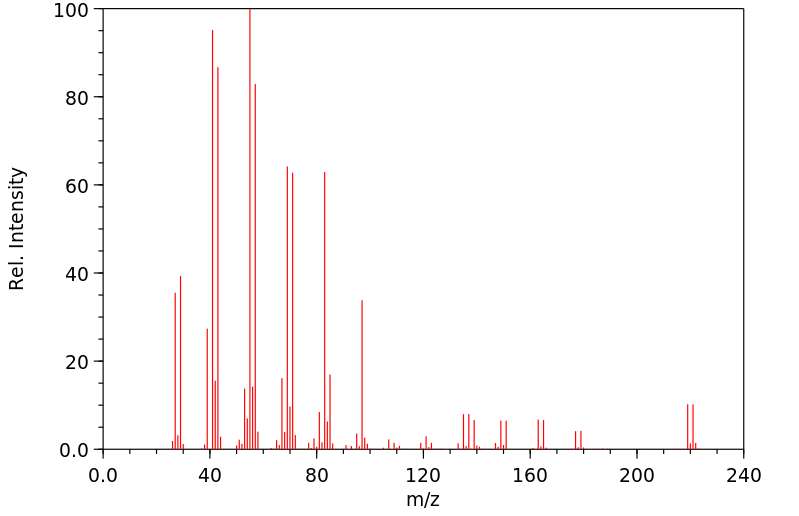
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4