扑米酮 | 125-33-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:281-282°C
-
沸点:358.94°C (rough estimate)
-
密度:1.1402 (rough estimate)
-
闪点:9℃
-
溶解度:极微溶于水,微溶于乙醇(96%)。它溶于碱性溶液。
-
物理描述:Primaclone is an odorless white crystalline powder. Slightly bitter taste. No acidic properties. (NTP, 1992)
-
颜色/状态:Crystals
-
气味:Odorless
-
味道:Practically tasteless
-
蒸汽压力:3.3X10-10 mm Hg at 25 °C (est)
-
水溶性:-2.64
-
稳定性/保质期:
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
解离常数:pKa = 12.3 (acidic character of ionizable functional group)
-
碰撞截面:146.9 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
保留指数:2250;2239;2242;2243;2202;2244.7;2227.2;2200;2225;2247;2248.7
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:2
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S22,S36,S45
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:29335990
-
危险品运输编号:3249
-
RTECS号:UV9100000
-
包装等级:III
-
危险类别:6.1(b)
-
储存条件:贮存时应采取密封措施。
SDS
模块 1. 化学品
产品名称: Primidone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吞咽有害。
防范说明
[预防] 使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
[急救措施] 食入:若感不适,呼叫解毒中心/医生。漱口。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 普里米酮
百分比: >98.0%(LC)(N)
CAS编码: 125-33-7
俗名: 5-Ethyl-5-phenylhexahydropyrimidine-4,6-dione , 2-Deoxyphenobarbital
分子式: C12H14N2O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
普里米酮 修改号码:5
模块 4. 急救措施
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 281°C
普里米酮 修改号码:5
模块 9. 理化特性
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 不溶于
[其他溶剂]
溶于: 二甲基甲酰胺
微溶于: 吡啶
极微溶于: 乙醇
不溶于: 醚
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: orl-rat LD50:1500 mg/kg
ipr-rat LD50:240 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: mmo-sat 6666 ug/plate (+S9)
cyt-hmn-leu 1 mg/L
致癌性: orl-mus TDLo:18200 mg/kg/2Y-C
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: UV9100000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
普里米酮 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
扑米酮 属于巴比妥类抗癫痫药,其抗癫痫作用类似于苯巴比妥,对癫痫大发作及精神运动性发作有效。在体内转化为具有抗惊厥作用的苯巴比妥和苯乙基丙二酰胺,二者均具抗癫痫作用,但效果不如苯妥英钠,可与苯妥英钠合用以增强其疗效。扑米酮对控制强直性和阵挛性癫痫优于苯巴比妥。
1954年,扑米酮获得美国FDA审批上市,并持续被临床使用。普萘洛尔和扑米酮是首选的循证药物,可用作初始单药治疗,两药联用有时会产生加性作用。
药理作用扑米酮在体内的主要代谢产物为苯巴比妥,与其共同发挥作用。体外电生理实验显示,该药物使神经细胞的氯离子通道开放,导致细胞去极化,拟似γ-氨基丁酸(GABA)的作用。在治疗浓度时可降低谷氨酸的兴奋作用,加强γ-氨基丁酸的抑制作用,抑制中枢神经系统单突触和多突触传递,导致整个神经细胞兴奋性降低,提高运动皮质电刺激阈,使发作阈值提高,还可以抑制致痫灶放电的传播。
药动学口服易吸收,成人tmax为2.7~5.2小时,儿童为4~6小时。生物利用度高达92%。分布广泛,可通过胎盘和乳汁分泌。Vd为0.64~0.72L/kg,血浆蛋白结合率较低。半衰期约10小时。主要通过肝脏代谢,经肾脏排泄。
适应证扑米酮(扑痫酮)是一种抗癫痫药物,用于治疗癫痫强直-阵挛性发作(大发作)、单纯部分性发作和复杂部分性发作的单药或联合用药治疗。它也适用于特发性震颤和老年性震颤的治疗,对于特发性震颤患者的手部震颤治疗效果比较明显,能降低手部震颤的幅度,减少约50%的幅度震颤。对于从未接受过治疗或已用过普萘洛尔的病人,扑米酮的剂量可显著减少震颤幅度。
不良反应扑米酮治疗中可能会有一些不良反应,如头昏、恶心和呕吐等。随着用药时间延长,症状可能减轻或达到耐受。其他常见副作用包括视力改变、复视、眼球震颤、共济失调、认识障碍、焦虑、抑郁以及皮肤过敏反应。
药物相互作用扑米酮与多种药物存在相互作用,例如:
用药过量过量可能会产生结晶尿,其可能与血清中扑米酮的浓度超过80μg/mL以及肾损害有关。处理方法是对症支持治疗。
生物活性Primidone (NCI-C56360) 是一种嘧啶二酮类抗惊厥药。
靶点- 钠通道
白色结晶性粉末,熔点281-282℃。微溶于醇,几乎不溶于水、丙酮或苯。无臭,味微苦。
用途用于癫痫大发作和精神运动性发作。抗癫痫作用与苯巴比妥近似,但比苯巴比妥有选择性;作用强度不如苯妥英钠,但两者合用有协效应。
生产方法将乙酸乙酯加入乙醇钠中,回流半小时后冷却至室温。加入乙基苯基丙二酸二乙酯及硫脲,加流9小时。冷至0℃,加入乙醇,在0-5℃滴入盐酸至pH=3-3.5。加入锌粉,升温至回流,缓缓补加盐酸保持的应液有2-3%含酸量,加毕,保温3小时。趁热过滤,滤液回收乙醇。冷至20℃,加水过滤,洗涤滤饼,得扑米酮。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯巴比妥 phenobarbital 50-06-6 C12H12N2O3 232.239 5-苯基-5-乙基-2-硫代硫巴比妥酸 5-ethyl-5-phenyl-2-thiobarbituric acid 2753-74-4 C12H12N2O2S 248.305 2-乙基-2-苯基丙二酰胺 2-ethyl-2-phenylmalonamide 7206-76-0 C11H14N2O2 206.244 5-乙基-5-苯基-4,6(1H,5H)-嘧啶二酮 5-ethyl-5-phenyl-1H-pyrimidine-4,6-dione 694447-38-6 C12H12N2O2 216.239 —— 4-thiophenobarbital 60024-01-3 C12H12N2O2S 248.305 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯巴比妥 phenobarbital 50-06-6 C12H12N2O3 232.239 —— 5-ethyldihydro-1-methyl-5-phenyl-4,6(1H,5H)-pyrimidinedione 69243-48-7 C13H16N2O2 232.282 —— 2-hydroxyprimidone 75524-08-2 C12H14N2O3 234.255 5-乙基-1,3-二甲基-5-苯基-二氢-嘧啶-4,6-二酮 5-ethyl-1,3-dimethyl-5-phenyl-dihydro-pyrimidine-4,6-dione 104169-76-8 C14H18N2O2 246.309 —— 1,5-diethyl-5-phenyl-dihydro-pyrimidine-4,6-dione 107480-93-3 C14H18N2O2 246.309 2-乙基-2-苯基丙二酰胺 2-ethyl-2-phenylmalonamide 7206-76-0 C11H14N2O2 206.244
反应信息
-
作为反应物:描述:参考文献:名称:New chemical aspects of primidone metabolism摘要:DOI:10.1016/0223-5234(90)90165-y
-
作为产物:参考文献:名称:Boon et al., Journal of the Chemical Society, 1954, p. 3263,3268摘要:DOI:
文献信息
-
[EN] PROCESSES USEFUL FOR THE SYNTHESIS OF (R)-1-{2-[4'-(3-METHOXYPROPANE-1-SULFONYL)-BIPHENYL-4-YL]-ETHYL}-2-METHYL-PYRROLIDINE<br/>[FR] PROCÉDÉS UTILES POUR LA SYNTHÈSE DE LA (R)-1-{2-[4'-(3-MÉTHOXYPROPANE-1-SULFONYL)-BIPHÉNYL-4-YL]-ÉTHYL}-2-MÉTHYL-PYRROLIDINE申请人:ARENA PHARM INC公开号:WO2009128907A1公开(公告)日:2009-10-22Processes useful for making a pharmaceutically useful compound according to Formula (I), forms of such a compound, and intermediates useful in such processes are described.根据公式(I)制备药用化合物的有用过程,以及该化合物的形式和在这些过程中有用的中间体被描述。
-
Substituted 1,3-thiazole compounds, their production and use申请人:——公开号:US20040053973A1公开(公告)日:2004-03-18(1) A 1,3-thiazole compound of which the 5-position is substituted with a 4-pyridyl group having a substituent including no aromatic group or (2) a 1,3-thiazole compound of which the 5-position is substituted with a pyridyl group having at the position adjacent to a nitrogen atom of the pyridyl group a substituent including no aromatic group has an excellent p38 MAP kinase inhibitory activity.
-
Piperidine derivatives申请人:——公开号:US20040142956A1公开(公告)日:2004-07-22Compounds, compositions and methods are provided that are useful in the treatment or prevention of conditions or disorders associated with a neuropeptide receptor. The subject methods are particularly useful in the treatment and/or prevention of endocrine, metabolic, cardiovascular, neurologic, psychiatric, gastrointestinal, genitourinary and other disorders.提供了在治疗或预防与神经肽受体相关的疾病或疾病的化合物、组合物和方法。这些方法特别适用于治疗和/或预防内分泌、代谢、心血管、神经、精神、消化、泌尿生殖和其他疾病。
-
ANTHELMINTIC COMPOUNDS AND COMPOSITIONS AND METHOD OF USING THEREOF申请人:Meng Charles Q.公开号:US20140142114A1公开(公告)日:2014-05-22The present invention relates to novel anthelmintic compounds of formula (I) below: wherein Y and Z are independently a bicyclic carbocyclic or a bicyclic heterocyclic group, or one of Y or Z is a bicyclic carbocyclic or a bicyclic heterocyclic group and the other of Y or Z is alkyl, alkenyl, alkynyl, cycloalkyl, phenyl, heterocyclyl or heteroaryl, and variables X 1 , X 2 , X 3 , X 4 , X 5 , X 6 , X 7 and X 8 are as defined herein. The invention also provides for veterinary compositions comprising the anthelmintic compounds of the invention, and their uses for the treatment and prevention of parasitic infections in animals.本发明涉及以下式(I)的新型驱虫化合物: 其中 Y和Z分别是双环碳环或双环杂环基团,或者Y或Z中的一个是双环碳环或双环杂环基团,另一个是烷基,烯基,炔基,环烷基,苯基,杂环基或杂芳基,以及变量X 1 ,X 2 ,X 3 ,X 4 ,X 5 ,X 6 ,X 7 和X 8 如本文所定义。本发明还提供了包含本发明的驱虫化合物的兽药组合物,以及它们用于治疗和预防动物寄生虫感染的用途。
-
Novel Bicyclic Pyridinones申请人:Pettersson Martin Youngjin公开号:US20120252758A1公开(公告)日:2012-10-04Compounds and pharmaceutically acceptable salts of the compounds are disclosed, wherein the compounds have the structure of Formula I as defined herein. Corresponding pharmaceutical compositions, methods of treatment, methods of synthesis, and intermediates are also disclosed.所述化合物及其药用可接受的盐被披露,其中所述化合物具有如本文所定义的Formula I的结构。相应的药物组合物、治疗方法、合成方法和中间体也被披露。
表征谱图
-
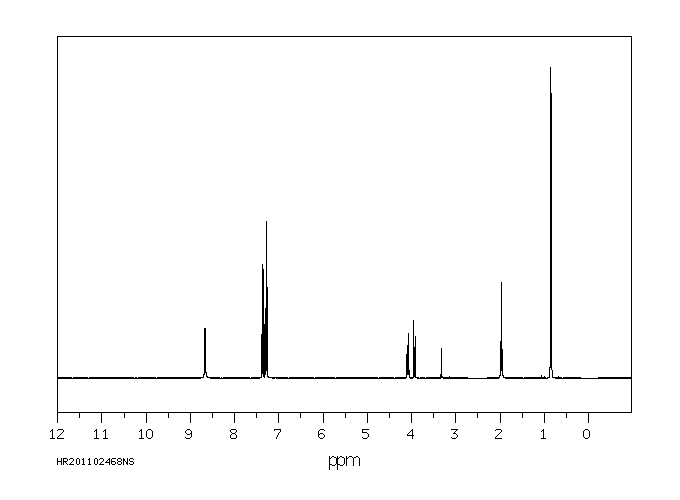
氢谱1HNMR
-
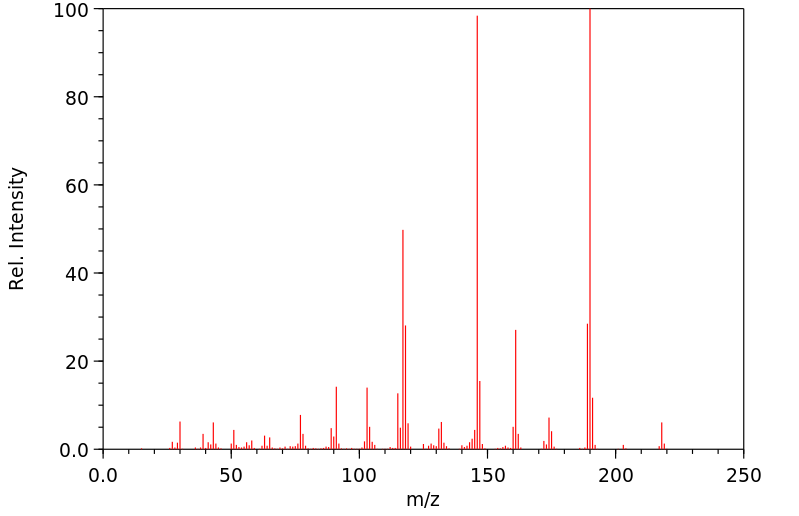
质谱MS
-
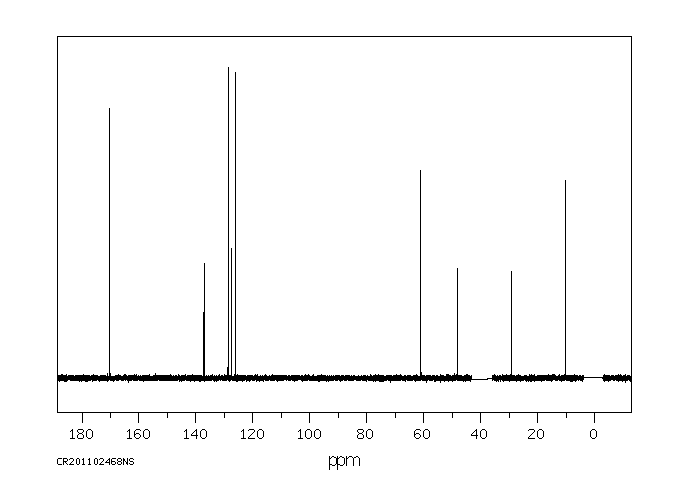
碳谱13CNMR
-
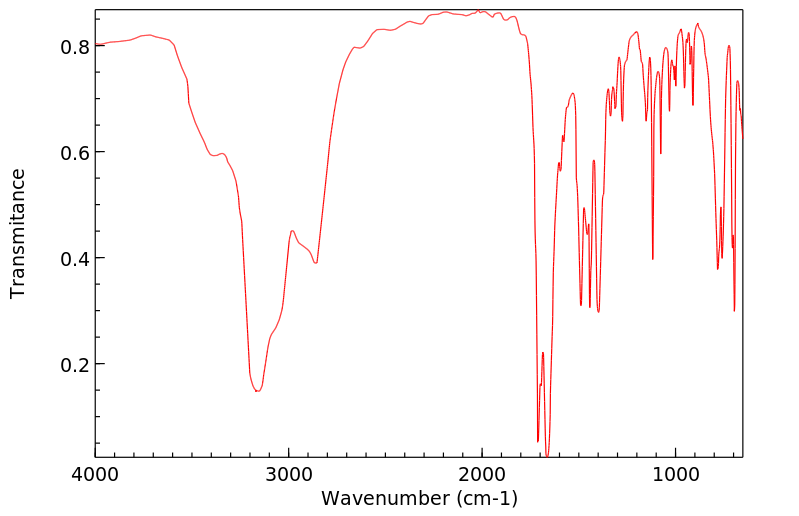
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息