1,8-双(溴甲基)萘 | 2025-95-8
中文名称
1,8-双(溴甲基)萘
中文别名
1,8-二(溴甲基)萘
英文名称
1,8-bis(bromomethyl)naphthalene
英文别名
1,8-di(bromomethyl)naphthalene
CAS
2025-95-8
化学式
C12H10Br2
mdl
——
分子量
314.019
InChiKey
GCZOMCDXYFMAGP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:131-133 °C (lit.)
-
沸点:386.3±22.0 °C(Predicted)
-
密度:1.720±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下稳定。
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S27,S28,S36/37/39,S45
-
危险类别码:R34
-
海关编码:2903999090
-
包装等级:III
-
WGK Germany:3
-
危险品运输编号:UN 3261
-
储存条件:常温下应存放在阴凉通风的地方。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 1,8-Bis(bromomethyl)naphthalene
CAS-No. : 2025-95-8
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Skin corrosion (Category 1B)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Causes burns.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Danger
Hazard statement(s)
H314 Causes severe skin burns and eye damage.
Precautionary statement(s)
P280 Wear protective gloves/ protective clothing/ eye protection/ face
protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
P310 Immediately call a POISON CENTER or doctor/ physician.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R34 Causes burns.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S27 Take off immediately all contaminated clothing.
S28 After contact with skin, wash immediately with plenty of water.
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
S45 In case of accident or if you feel unwell, seek medical advice immediately
(show the label where possible).
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C12H10Br2
Molecular Weight : 314,02 g/mol
Component Concentration
1,8-Bis(bromomethyl)naphthalene
CAS-No. 2025-95-8 -
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Take off contaminated clothing and shoes immediately. Wash off with soap and plenty of water. Consult a
physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes, and
skin., spasm, inflammation and edema of the larynx, spasm, inflammation and edema of the bronchi,
pneumonitis, pulmonary edema, burning sensation, Cough, wheezing, laryngitis, Shortness of breath,
Headache, Nausea
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Hydrogen bromide gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle
respirator type N100 (US) or type P3 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use
respirators and components tested and approved under appropriate government standards such
as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: crystalline
Colour: light yellow
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 131 - 133 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents, Strong bases
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Material is extremely destructive to the tissue of
the mucous membranes and upper respiratory tract.
Ingestion May be harmful if swallowed. Causes burns.
Skin May be harmful if absorbed through skin. Causes skin burns.
Eyes Causes eye burns.
Signs and Symptoms of Exposure
Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes, and
skin., spasm, inflammation and edema of the larynx, spasm, inflammation and edema of the bronchi,
pneumonitis, pulmonary edema, burning sensation, Cough, wheezing, laryngitis, Shortness of breath,
Headache, Nausea
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: 3261 IMDG: 3261 IATA: 3261
UN proper shipping name
ADR/RID: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (1,8-Bis(bromomethyl)naphthalene)
IMDG: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S. (1,8-Bis(bromomethyl)naphthalene)
IATA: Corrosive solid, acidic, organic, n.o.s. (1,8-Bis(bromomethyl)naphthalene)
Transport hazard class(es)
ADR/RID: 8 IMDG: 8 IATA: 8
Packaging group
ADR/RID: II IMDG: II IATA: II
Environmental hazards
ADR/RID: no IMDG Marine Pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,8-二甲基萘 1,8-dimethylnaphthalene 569-41-5 C12H12 156.227 1,8-萘二甲醇 naphthalene-1,8-diyldimethanol 2026-08-6 C12H12O2 188.226 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,8-二甲基萘 1,8-dimethylnaphthalene 569-41-5 C12H12 156.227 —— 1-bromomethyl-8-t-butylperoxymethylnaphthalene 93131-88-5 C16H19BrO2 323.23 1,8-二乙烯基萘 1,8-divinylnaphthalene 17935-66-9 C14H12 180.249 —— 1,8-Diethyl-naphthalin 17935-68-1 C14H16 184.281 1,8-二乙炔基-萘 1,8-diethynylnaphthalene 18067-44-2 C14H8 176.218 [8-(硫烷基甲基)萘-1-基]甲硫醇 1,8-bis(mercaptomethyl)naphthalene 60948-99-4 C12H12S2 220.359
反应信息
-
作为反应物:描述:参考文献:名称:1,8-双(溴甲基)萘在激光中的双光子化学:通过分子内CC键形成生成Generation摘要:以二苯甲酮为敏化剂的氩离子激光诱导的1,8-双(溴甲基)萘(1)在激光射流中的光解得到 通过中间非Kekulé双自由基1,8-二甲基对苯二环的环化,将8%(61%的转化率)的ena(4);在此双光子过程中,第一个光子(333 nm)消耗1,而第二个光子(351和364 nm)形成2,两个步骤均被二苯甲酮敏化。DOI:10.1016/s0040-4039(00)74165-7
-
作为产物:描述:参考文献:名称:A New Synthesis of Acenaphthene摘要:DOI:10.1021/ja01107a501
文献信息
-
Synthesis of binuclear iridium(III) and rhodium(III) complexes bearing methylnaphthalene-linked N-heterocyclic carbenes, and application to intramolecular hydroamination作者:Kenichi Ogata、Toshinori Nagaya、Shin-ichi FukuzawaDOI:10.1016/j.jorganchem.2010.04.006日期:2010.6with silver oxide followed by [Cp∗MCl2]2 (M = Ir, Rh), binuclear iridium and rhodium complexes 2 were formed. Reaction of these complexes 2 with AgPF6 afforded Cl-bridged cationic binuclear iridium and rhodium complexes 3. X-ray crystallographic analysis of 3 revealed that the two imidazole rings of the carbene ligand are in a parallel geometry. The cationic binuclear iridium complexes 3-Ir could be
-
Mononuclear and Polynuclear Chain Complexes of a Series of Multinucleating N/S Donor Ligands作者:Tanya K. Ronson、Harry Adams、Michael D. WardDOI:10.1002/ejic.200500704日期:2005.11chelating arms derived from 3-[2-(methylsulfanyl)phenyl]pyrazole linked to central aromatic spacers by methylene units. Complexes with a variety of architectures have been obtained, including simple mononuclear complexes and polynuclear chain complexes. The p-xylyl-spaced ligand L1 forms one-dimensional helical coordination polymers with copper(I) and silver(I) ions. These polymers display interligand我们已经制备了一系列五个配体,其潜在的 N,S-双齿螯合臂衍生自 3-[2-(甲基硫烷基)苯基]吡唑,通过亚甲基单元与中心芳族间隔基相连。已经获得了具有多种结构的配合物,包括简单的单核配合物和多核链配合物。对二甲苯基间隔的配体 L1 与铜 (I) 和银 (I) 离子形成一维螺旋配位聚合物。这些聚合物在每个螺旋链中显示出配位间芳族堆积相互作用。间二甲苯基间隔的配体 L2 与铜 (I) 形成配位聚合物,但与较大的银 (I) 离子形成单核配合物,其中中心苯环参与 η1 π 型 Ag…C 与AgI。3、3'-联苯间隔配体 L3 也与银 (I) 和铜 (I) 离子形成一维聚合物,但在这种情况下,一个金属中心和下一个金属中心之间的桥接配体序列遵循锯齿形路径而不是呈螺旋状。1,8-萘基间隔的配体 L4 仅与铜 (I) 和银 (I) 离子形成单核配合物,表明该间隔基不够大,无法强制桥接配位模式。由 2,4
-
Synthesis and solid state structures of N,N′-linked carbazoles and indoles作者:Jason Bloxham、Christopher J Moody、Alexandra M.Z SlawinDOI:10.1016/s0040-4020(02)00344-7日期:2002.5The synthesis and solid state conformations of a number of N,N′-linked carbazoles and indoles has been investigated. Using xylyl-based linkers, the o, m and p-linked carbazoles and indoles 4–6, 8–10 and 12, 13 were prepared. Likewise, unsymmetrical derivatives were also prepared. Investigation using X-ray crystallography showed that the heterocycles adopted either stretched or folded conformations
-
Dearomatizing Spiroannulation Reagents: Direct Access to Spirocycles from Indoles and Dihalides作者:John T. R. Liddon、James A. Rossi-Ashton、Richard J. K. Taylor、William P. UnsworthDOI:10.1021/acs.orglett.8b01248日期:2018.6.1Unfunctionalized indoles can be directly converted into 3,3′-spirocyclic indolenines and indolines upon reaction with electrophilic dihalides in the presence of t-BuOK/BEt3. This double C–C bond forming reaction, which simultaneously generates a quaternary spirocyclic center, typically proceeds in high yield and has good functional group tolerance. In contrast to existing dearomatizing spirocyclization
-
ELECTROCHROMIC SINGLE AND TWO-CORE VIOLOGENS AND OPTICAL ARTICLES CONTAINING THEM申请人:ESSIL OR INTERNATIONAL (COMPAGNIE GENERALE D'OPTIQUE公开号:US20160231635A1公开(公告)日:2016-08-11The present invention relates to a group of novel electrochromic materials. More specifically, it relates to electrochromic materials based on either single or two-core viologen systems and the use of these viologen systems as a variable transmittance medium for the manufacture of an optical article, such as an ophthalmic lens.本发明涉及一组新颖的电致变色材料。更具体地说,它涉及基于单一或双核心紫罗兰体系的电致变色材料,以及将这些紫罗兰体系用作可变透射率介质,用于制造光学产品,例如眼镜片。
表征谱图
-
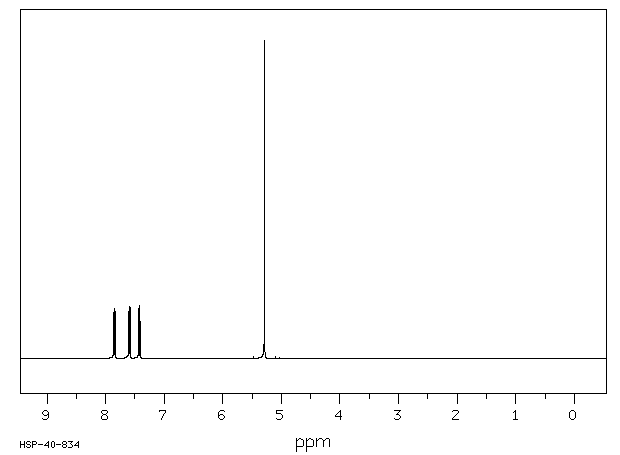
氢谱1HNMR
-
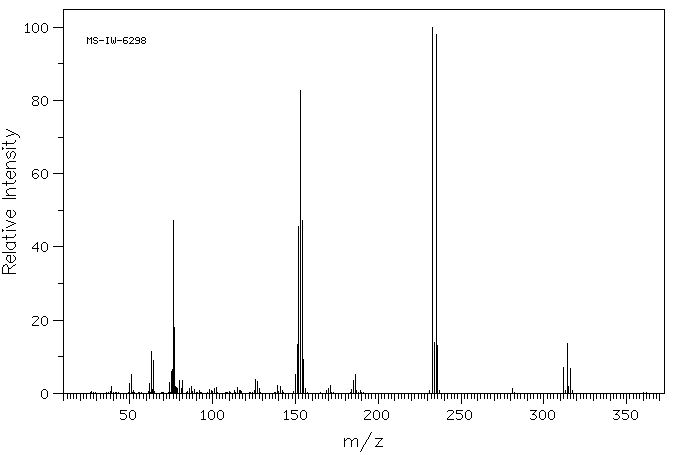
质谱MS
-
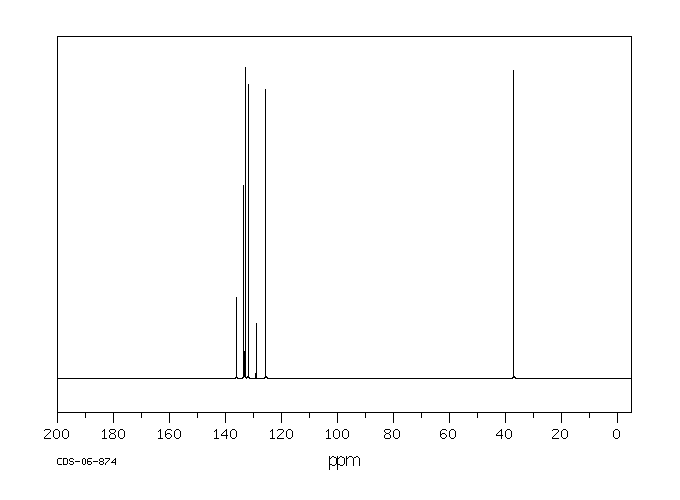
碳谱13CNMR
-
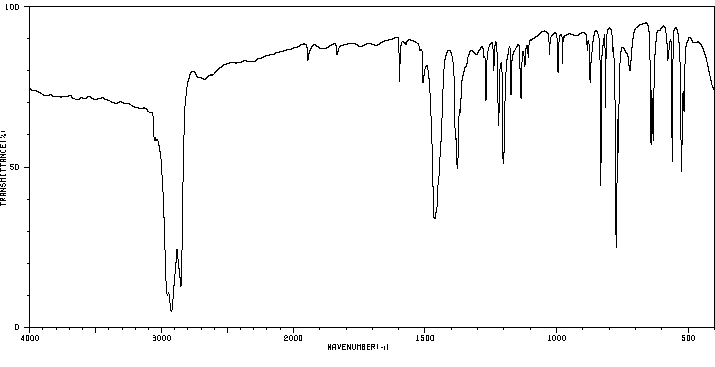
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮