1-(3-溴-4-甲氧基苯基)乙烯酮 | 35310-75-9
中文名称
1-(3-溴-4-甲氧基苯基)乙烯酮
中文别名
3'-溴-4'-甲氧基苯乙酮;3-溴-4-甲氧基苯乙酮
英文名称
3-bromo-4-methoxyacetophenone
英文别名
1-(3-bromo-4-methoxyphenyl)ethanone;1-(3-bromo-4-methoxyphenyl)ethan-1-one;3'-Brom-4'-methoxyacetophenon
CAS
35310-75-9
化学式
C9H9BrO2
mdl
MFCD01812494
分子量
229.073
InChiKey
JYPGOBDETCKKKV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:84-86°C
-
密度:1.421
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
海关编码:2914700090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:室温且干燥
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-(3-Bromo-4-methoxyphenyl)ethanone
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-(3-Bromo-4-methoxyphenyl)ethanone
CAS number: 35310-75-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
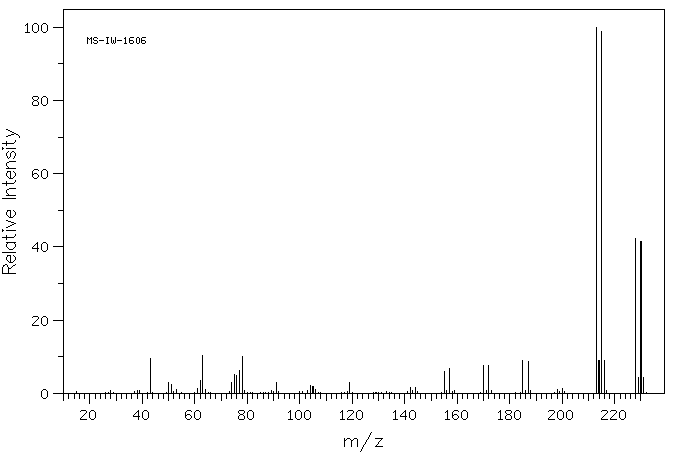
Molecular formula: C9H9BrO2
Molecular weight: 229.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-(3-Bromo-4-methoxyphenyl)ethanone
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-(3-Bromo-4-methoxyphenyl)ethanone
CAS number: 35310-75-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H9BrO2
Molecular weight: 229.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(3-溴-4-甲氧基苯基)乙醇 3-bromo-α-methyl-4-methoxybenzenemethanol 94670-25-4 C9H11BrO2 231.089 对甲氧基苯乙酮 1-(4-methoxyphenyl)ethanone 100-06-1 C9H10O2 150.177 3,4-二溴苯乙酮 3,4-Dibromacetophenon 3114-30-5 C8H6Br2O 277.943 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(3-bromo-4-methoxyphenyl)-2-oxoacetaldehyde —— C9H7BrO3 243.057 2-溴-1-(3-溴-4-甲氧基苯基)乙酮 2-bromo-1-(3-bromo-4-methoxyphenyl)ethanone 6096-83-9 C9H8Br2O2 307.969 1-(3-溴-4-羟基苯基)乙酮 3-bromo-4-hydroxyacetophenone 1836-06-2 C8H7BrO2 215.046 1-(3-溴-4-甲氧基苯基)乙醇 3-bromo-α-methyl-4-methoxybenzenemethanol 94670-25-4 C9H11BrO2 231.089 1-(5-溴-2-氟-4-甲氧基-苯基)-乙酮 1-(5-bromo-2-fluoro-4-methoxyphenyl)ethanone 914221-54-8 C9H8BrFO2 247.064 3-溴-4-甲氧基苯甲酸 3-bromo-4-methoxybenzoic acid 99-58-1 C8H7BrO3 231.046 3-溴-4-甲氧基苯甲酰胺 3-bromo-4-methoxybenzamide 200956-55-4 C8H8BrNO2 230.061 —— 1-(3-bromo-4-methoxyphenyl)-2-(3-methoxyphenylthio)ethanone 1156766-40-3 C16H15BrO3S 367.263 —— 2-(3-bromo-4-methoxyphenyl)-2-methyl-1,3-dioxolane 81224-34-2 C11H13BrO3 273.126
反应信息
-
作为反应物:描述:参考文献:名称:3-溴-5-(2-乙基咪唑并[1,2-a]吡啶-3-羰基)-2-羟基苯甲腈的合成摘要:本发明公开了一种3‑溴‑5‑(2‑乙基咪唑并[1,2‑a]吡啶‑3‑羰基)‑2‑羟基苯甲腈的合成方法,特别是涉及一种式(III)化合物的合成方法,具体涉及步骤A或者步骤B;步骤A:式(I)化合物与式(II)化合物先在有机溶剂中加热进行反应,所得反应产物与碱再在水存在的条件下加热继续反应,得到式(III)化合物;步骤B:式(I)化合物与式(II)化合物和碱先在有机溶剂中加热进行反应,所得反应产物再在水存在的条件下加热继续反应,得到式(III)化合物。公开号:CN111410654B
-
作为产物:描述:参考文献:名称:N-溴代琥珀酰亚胺碘催化芳基醚芳族溴化反应的机理摘要:尽管近年来碘催化的反应发展迅速,但是碘催化溴化的例子很少,这些反应的机理尚不清楚。在本文中,我们报道了NBS的I 2催化的芳基醚的芳族溴化反应,并详细介绍了包括动力学研究和动力学同位素效应在内的机理研究。研究表明,该反应实际上是由诱导期形成的IBr催化的,而决定速率的步骤是在IBr辅助下消除Wheland中间体的HBr。DOI:10.1016/j.tet.2017.10.073
文献信息
-
Direct Transformation of Ethylarenes into Primary Aromatic Amides with <i>N</i>-Bromosuccinimide and I<sub>2</sub>–Aqueous NH<sub>3</sub>作者:Shohei Shimokawa、Yuhsuke Kawagoe、Katsuhiko Moriyama、Hideo TogoDOI:10.1021/acs.orglett.6b00048日期:2016.2.19α-bromomethyl ketones and/or aryl methyl ketones were formed at the first reaction step and their iodoform-type reaction occurred at the second reaction step to provide primary aromatic amides. The present reaction is a useful and practical transition-metal-free method for the preparation of primary aromatic amides from ethylarenes.
-
Access to multi-functionalized oxazolines via silver-catalyzed heteroannulation of enamides with sulfoxonium ylides作者:Rui-Hua Liu、Qi-Chao Shan、Ya Gao、Teck-Peng Loh、Xu-Hong HuDOI:10.1016/j.cclet.2020.10.007日期:2021.4efficient Ag-catalyzed [4 + 1] heteroannulation reaction of enamides with α-carbonyl sulfoxonium ylides. The diastereoselective transformation provides a practical access to a diverse range of multi-functionalized oxazoline derivatives. The synthetic utility of the resultant tetra-substituted oxazolines is further demonstrated by a series of useful manipulations into valuable building blocks of pharmaceutical
-
Electrooxidation of Alcohols in an<i>N</i>-Oxyl-Immobilized Poly(ethylene-<i>co</i>-acrylic acid)/Water Disperse System作者:Hideo Tanaka、Jun Kubota、Seiji Miyahara、Manabu KuroboshiDOI:10.1246/bcsj.78.1677日期:2005.9The electrooxidation of alcohols in a disperse system with N-oxyl-immobilized polymer particles (PE-co-AA-N-Oxyl), prepared from poly(ethylene-co-acrylic acid) (PE-co-AA-CO2H), as a disperse phase and an aqueous saturated NaHCO3 containing 20 wt % NaBr as a disperse medium was successfully performed in a simple beaker-type undivided cell under constant-current conditions. The electrooxidation of alcohols proceeded in a similar manner by the use of PE-co-AA-CO2H as a disperse phase, though the current efficiency decreased in some extent. Protection of most of the carboxylic acid moieties on the surface of PE-co-AA-CO2H by esterification or amidation resulted in significant decrease of the product yields, suggesting that the carboxylic acid moieties on the surface of PE-co-AA-CO2H would also act as a mediator for the electrooxidation of alcohols. Both the recovered PE-co-AA-N-Oxyl and the aqueous solution were used repeatedly for the electrooxidation of alcohols, thereby offering a formally closed system for the electrooxidation of alcohols.在采用N-氧基固定化聚合物颗粒(PE-co-AA-N-Oxyl)作为分散相,以及含有20 wt% NaBr的饱和NaHCO3水溶液作为分散介质的分散体系中,成功地在一简单的杯型无隔膜电池中在恒电流条件下进行了醇的电氧化。通过使用PE-co-AA-CO2H作为分散相,醇的电氧化过程与此类似,尽管电流效率在某种程度上有所降低。通过酯化或酰胺化保护PE-co-AA-CO2H表面上的大多数羧酸官能团导致产品收率显著降低,表明PE-co-AA-CO2H表面上的羧酸官能团也会作为醇电氧化的介体。回收的PE-co-AA-N-Oxyl和水溶液均可重复用于醇的电氧化,从而为醇的电氧化提供了一个形式上的封闭系统。
-
Electrooxidation of Alcohols in <i>N</i>-Oxyl-Immobilized Silica Gel/Water Disperse System: Approach to Totally Closed System作者:Manabu Kuroboshi、Kentaro Goto、Hideo TanakaDOI:10.1055/s-0028-1087987日期:——Electrooxidation of alcohols in a disperse system with N-oxyl-immobilized silica gel as a disperse phase and an aqueous NaBr/NaHCO3 solution as a disperse medium proceeded smoothly to give the corresponding carbonyl compounds. The N-oxyl-immobilized silica gel 1 was prepared by silane-coupling method. Recycling of both the aqueous solution and the N-oxyl-immobilized silica gel 1 was performed successfully.
-
Synthesis, biological evaluation and molecular modeling studies of psammaplin A and its analogs as potent histone deacetylases inhibitors and cytotoxic agents作者:Jiachen Wen、Yu Bao、Qun Niu、Jiang Liu、Jinyu Yang、Wanqiao Wang、Tao Jiang、Yinbo Fan、Kun Li、Jian Wang、Linxiang Zhao、Dan LiuDOI:10.1016/j.bmcl.2015.12.094日期:2016.9In this study, a concise synthetic method of psammaplin A was achieved from 3-bromo-4-hydroxybenzaldahyde and hydantoin through a four-step synthesis via Knoevenagel condensation, hydrolysis, oximation and amidation in 37% overall yield. A collection of novel psammaplin A analogs focused on the variations of substituents at the benzene ring and modifications at the oxime moiety were synthesized. Among
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
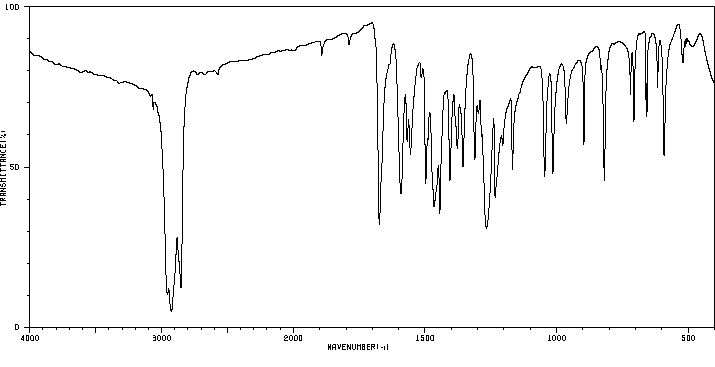
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷