正庚醚 | 629-64-1
中文名称
正庚醚
中文别名
二庚醚;1-庚氧庚烷;庚醚;二庚基醚
英文名称
Diheptyl ether
英文别名
Heptyl ether;1-heptoxyheptane
CAS
629-64-1
化学式
C14H30O
mdl
MFCD00015288
分子量
214.392
InChiKey
UJEGHEMJVNQWOJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-25.3°C (estimate)
-
沸点:262 °C
-
密度:0.80
-
保留指数:1458
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:15
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
RTECS号:MK1390500
-
储存条件:存放在密封容器内,并放置在阴凉、干燥处。请确保储存地点远离氧化剂。
SDS
Heptyl Ether Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Heptyl Ether
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Heptyl Ether
Percent: >98.0%(GC)
CAS Number: 629-64-1
Synonyms: Diheptyl Ether
Chemical Formula: C14H30O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Heptyl Ether
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
262°C
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
0.80
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Heptyl Ether
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: ivn-mus LD50:470 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
MK1390500
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Heptyl Ether
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Heptyl Ether
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Heptyl Ether
Percent: >98.0%(GC)
CAS Number: 629-64-1
Synonyms: Diheptyl Ether
Chemical Formula: C14H30O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Heptyl Ether
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
262°C
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
0.80
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Heptyl Ether
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: ivn-mus LD50:470 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
MK1390500
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Heptyl Ether
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:Hydroammonolysis of di-1-heptyl ether and the 1-heptyl ester of enanthic acid摘要:DOI:10.1007/bf00953364
-
作为产物:参考文献:名称:醇之间的有机卤化物催化的脱水O-烷基化:脂肪族醚合成的简便醚化方法摘要:有机卤化物有效地催化了醇之间的脱水O-烷基化反应,为制备有用的对称和不对称脂族醚提供了选择性,实用,绿色,易于扩展的均和交叉醚化方法。DOI:10.1039/c5gc00284b
文献信息
-
Thiourea-Mediated Halogenation of Alcohols作者:Amar R. Mohite、Ravindra S. Phatake、Pooja Dubey、Mohamed Agbaria、Alexander I. Shames、N. Gabriel Lemcoff、Ofer ReanyDOI:10.1021/acs.joc.0c01431日期:2020.10.16conditions expedited by the presence of substoichiometric amounts of thiourea additives is presented. The amount of thiourea added dictates the pathway of the reaction, which may diverge from the desired halogenation reaction toward oxidation of the alcohol, in the absence of thiourea, or toward starting material recovery when excess thiourea is used. Both bromination and chlorination were highly efficient
-
Chemical conversions using sheet silicates: novel intermolecular dehydrations of alcohols to ethers and polymers作者:James A. Ballantine、Mary Davies、Howard Purnell、Mongkon Rayanakorn、John M. Thomas、Kevin J. WilliamsDOI:10.1039/c39810000427日期:——Aliphatic primary alcohols, when intercalated in certain ion-exchanged montmorillonites, react preferentially via an intermolecular nucleophilic displacement of water to give high yields of di-(alk-1-yl) ethers, rather than the competitive intramolecular dehydration to alkenes; an essentially similar process yields polymeric material, poly(phenylenemethylene), from benzyl alcohol, but aliphatic secondary
-
Action des trialkylsilanes sur les aldehydes aliphatiques作者:Rolland Bourhis、Emile FrainnetDOI:10.1016/s0022-328x(00)89613-8日期:1975.2Two competing reactions ensue when trialkylsilanes HSiR′3 are treated with aliphatic aldehydes RCHO in the absence of a solvent and using a nickel catalyst (obtained by the treatment of anhydrous NiCl2 with a trialkylsilane); one gives an alcoxysilane RCH2OSiR′3, the other an ether oxide (RCH2)2O and a siloxane (R′3Si)2O. By variation of the reaction conditions, either reaction can be made exclusive
-
RADIATION-SENSITIVE RESIN COMPOSITION, RESIST PATTERN-FORMING METHOD, COMPOUND AND METHOD OF GENERATING ACID申请人:JSR CORPORATION公开号:US20200341376A1公开(公告)日:2020-10-29A radiation-sensitive resin composition contains: a polymer that includes a structural unit including an acid-labile group; and a radiation-sensitive acid generating agent. The radiation-sensitive acid generating agent includes a sulfonate anion and a radiation-sensitive cation. The sulfonate anion includes two or more rings, and an iodine atom and a monovalent group having 0 to 10 carbon atoms which includes at least one of an oxygen atom and a nitrogen atom bond to at least one of the two or more rings. The ring is preferably an aromatic ring. The radiation-sensitive acid generating agent is preferably a compound represented by formula (1). In the formula (1), A 1 represents a group obtained from a compound which includes a ring having 3 to 20 ring atoms by removing (p+q+r+1) hydrogen atoms on the ring.
-
Effect of Alcohol Structure on the Kinetics of Etherification and Dehydration over Tungstated Zirconia作者:Julie Rorrer、Suresh Pindi、F. Dean Toste、Alexis T. BellDOI:10.1002/cssc.201801067日期:2018.9.21energies for etherification and dehydration, demonstrating the possibility to produce both symmetrical and asymmetrical linear ethers. Reactions over a series of C6 alcohols with varying methyl branch positions indicated that substituted alcohols (2°, 3°) and alcohols with branches on the β‐carbon readily undergo dehydration, but alcohols with branches at least three carbons away from the ‐OH group are线性和支链醚分子作为可从生物质衍生的醇生产的柴油添加剂和润滑剂,最近引起了人们的兴趣。在这项研究中,钨酸锆被鉴定为是一种选择性的绿色固体酸催化剂,用于液相中伯醇的直接醚化,在393 K下对C 6 -C 12线性醇偶联的醚选择性达到> 94%。线性伯醇(C 6 –C 12)对醚化和脱水的表观活化能的影响可忽略不计,证明了同时生产对称和不对称线性醚的可能性。在一系列C 6上的反应甲基支链位置变化的醇表明,取代的醇(2°,3°)和在β-碳上具有支链的醇容易脱水,但是在支链上离OH基团至少三个碳的醇对醚具有高度选择性。合成并测试了一种新型的模型化合物4-己基-1-十二烷醇,以进一步证明这种结构-活性关系。醇结构对选择性的影响趋势与先前提出的醚化和脱水机理相一致,并有助于定义可能的途径,以从生物质衍生的醇中选择性地形成醚。
表征谱图
-
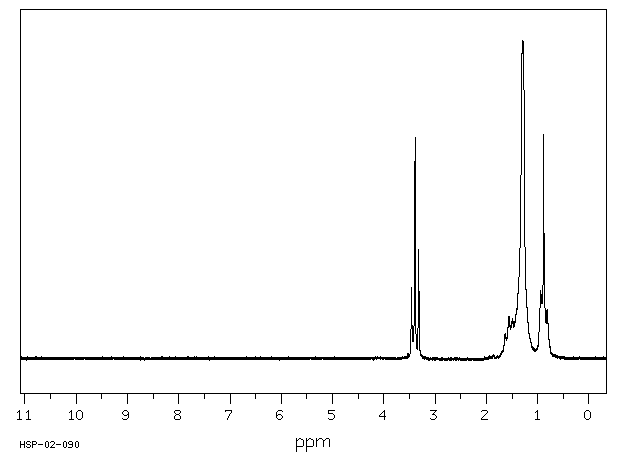
氢谱1HNMR
-
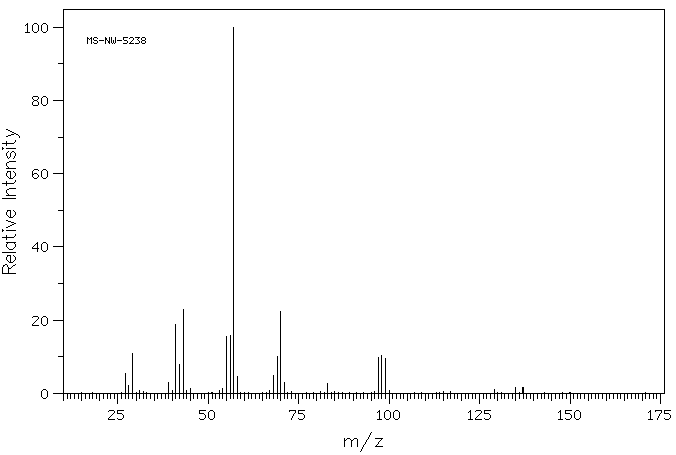
质谱MS
-
碳谱13CNMR
-
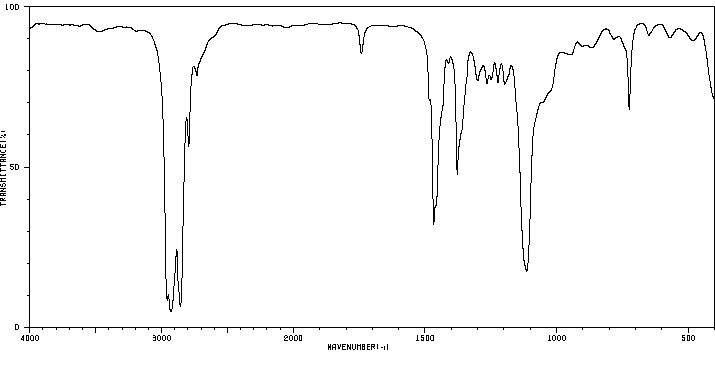
红外IR
-
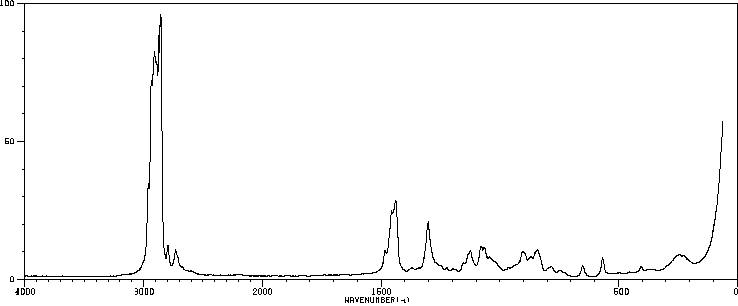
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷