1-辛烯-4-炔 | 24612-83-7
中文名称
1-辛烯-4-炔
中文别名
——
英文名称
1-octene-4-yne
英文别名
Oct-1-en-4-yne;1-octen-4-yne;Octen-(1)-in-(4)
CAS
24612-83-7
化学式
C8H12
mdl
MFCD00048793
分子量
108.183
InChiKey
ICBXOVYWGIDVQO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Narasimhan, S.; Swarnalakshmi, S.; Balakumar, R., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1998, vol. 37, # 11, p. 1189 - 1190摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 bis(acetylacetonate)nickel(II) 、 三苯基膦 作用下, 以 四氢呋喃 为溶剂, 以98%的产率得到1-辛烯-4-炔参考文献:名称:Cross coupling of magnesium diacetylenides with functional allylic and halide-containtng-compounds catalyzed by transition metal complexes摘要:DOI:10.1007/bf00952934
文献信息
-
Novel synthesis of an ω-alkynylorganometallic reagent via triple bond isomerization with potassium 3-aminopropylamide: ω-(9-borabicyclo[3.3.1]nonan-9-yl)alkl-1-ynes作者:Charles Allan Brown、Ei-ichi NegishiDOI:10.1039/c39770000318日期:——Isomerization of 1-(9-borabicyclo[3.3.1]nonan-9-yl)oct-4-yne, readily prepared by hydroboration of oct-1-en-4-yne with 9-borabicyclo[3.3.1]nonane and 2·5–3·0 equiv. of potassium 3-aminopropylamide, yields quantitatively 1-(9-borabicyclo[3.3.1]nonan-9-yl)oct-7-yne, which undergoes typical organoborane, reactions such as oxidation and alkyltransfer to α-bromoketones.
-
Some reactions and properties of molecular diatomic carbon C2. An experimental and theoretical treatment作者:Philip S. Skell、Lloyd M. Jackman、Sheikh Ahmed、Michael L. McKee、Philip B. ShevlinDOI:10.1021/ja00194a042日期:1989.6Ab initio calculations at the HF/3-21G level predict that both sup 1}Csub 2} and sup 3}Csub 2} will add to ethylene without barrier. At the MP2/6-31G*//3-21G level, the triplet adduct is calculated to be more stable than sup 3}Csub 2} and ethylene by 46.0 kcal/mol. The reactions of sup 1}Csub 2} and sup 3}Csub 2} with methane and hydrogen have also been investigated theoretically.双原子碳,Csub 2},在 77 K 的凝聚相中与丙烯和 (E)- 和 (Z)-2- 丁烯反应。产物可以通过涉及初始添加 Csub 2} 到烯烃生成 1,4-双自由基。然后,该双自由基可以提取氢或添加另一个烯烃分子以产生歧化为烯炔的 1,6-双自由基。因此,Csub 2} 与丙烯的反应产生 1-戊炔、3-甲基-1-丁炔、4-甲基庚-6-en-1-yne、6-甲基庚-1-en-4-yne 和 oct -1-en-4-yne。HF/3-21G 水平的从头算计算预测,sup 1}Csub 2} 和 sup 3}Csub 2} 都会无阻隔地添加到乙烯中。在 MP2/6-31G*//3-21G 水平,三线态加合物经计算比 sup 3}Csub 2} 和乙烯稳定 46.0 kcal/mol。
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Co: Org.Verb.2, 2.2.2.5, page 87 - 101作者:DOI:——日期:——
-
Hydroboration. 64. Effect of structure on the relative reactivity of representative alkenes and alkynes toward hydroboration by dibromoborane-methyl sulfide作者:Herbert C. Brown、J. ChandrasekharanDOI:10.1021/jo00153a004日期:1983.3
-
Reaction of acetylenes with chlorosulfonyl isocyanate作者:Emil J. Moriconi、Yasuo ShimakawaDOI:10.1021/jo00967a007日期:1972.1
表征谱图
-
氢谱1HNMR
-
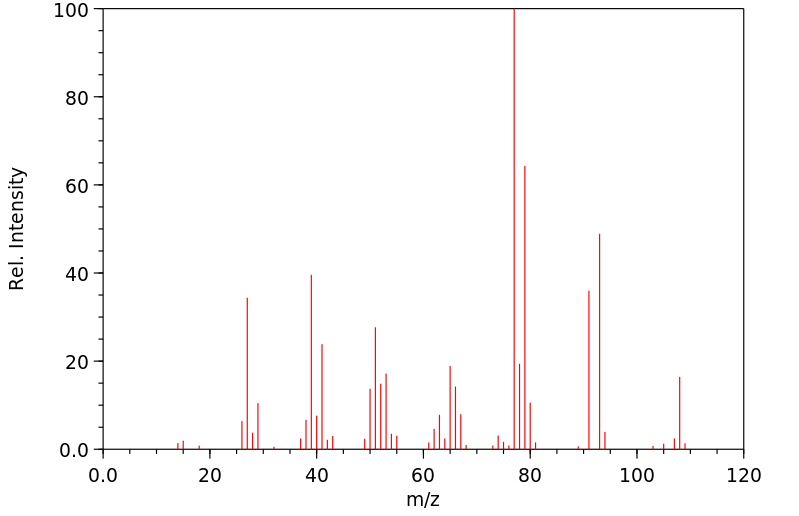
质谱MS
-
碳谱13CNMR
-
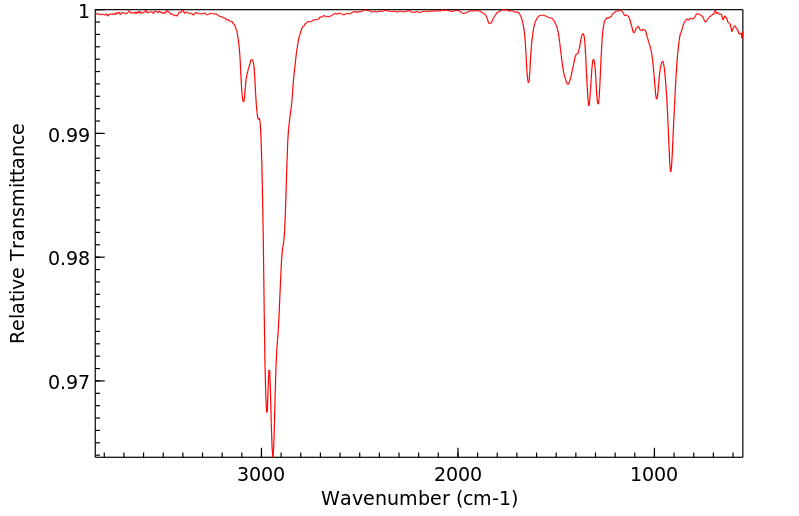
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-