2',4'-二氯苯戊酮 | 61023-66-3
中文名称
2',4'-二氯苯戊酮
中文别名
2',4'-二氯苯基-1-戊酮;2',4'-二氯代苯戊酮;2,4-二氯苯戊酮
英文名称
1-(2,4-dichlorophenyl)pentan-1-one
英文别名
1-(2,4-dichlorophenyl)-n-pentan-1-one;2',4'-Dichlorovalerophenone
CAS
61023-66-3
化学式
C11H12Cl2O
mdl
MFCD09028061
分子量
231.122
InChiKey
XVWXSWROOLWNCJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:297.3±20.0 °C(Predicted)
-
密度:1.20
-
稳定性/保质期:
避免接触氧化物
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
海关编码:2914700090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴-1-(2,4-二氯苯基)-1-戊酮 2-Bromo-1-(2,4-dichlorophenyl)pentan-1-one 86115-64-2 C11H11BrCl2O 310.018 —— 1-(2,4-Dichloro-phenyl)-2-methylene-pentan-1-one 93363-69-0 C12H12Cl2O 243.133 —— (2,4-Dichloro-phenyl)-(2-propyl-oxiranyl)-methanone 93363-70-3 C12H12Cl2O2 259.132
反应信息
-
作为反应物:描述:2',4'-二氯苯戊酮 在 sodium hydroxide 、 双氧水 作用下, 以 乙酸酐 、 丙酮 为溶剂, 反应 0.75h, 生成 (2,4-Dichloro-phenyl)-(2-propyl-oxiranyl)-methanone参考文献:名称:新型偶氮基丙醇酮及相关化合物的合成及口服抗真菌活性。摘要:为了找到口服活性抗真菌剂,合成了新的咪唑基-和1,2,4-三唑基丙醇酮I和相关化合物II-IV。化合物I衍生自酮V(方法A),α-二酮IX(方法B),α-羟基酮X(方法C),α-氯酮XII(方法D)和烯酮VI(方法E)。使用N,N'-羰基二咪唑,亚硫酰氯,N,N'-(硫代羰基)二咪唑,溴氯甲烷,2,2-二甲氧基丙烷和环己酮将由I与NaBH4合成的二元醇II环化为五元环状化合物III。二甲基缩酮。通过Grignard反应(方法F),X的羟甲基化(方法G)和酮XXI与1-[((三甲基甲硅烷基)甲基] -1,2,4-三唑(方法H)的反应,由I合成二元醇IV。通过评价肉汤对三种真菌的稀释MIC值和对白色念珠菌假菌丝体的抑制作用,检查了化合物I-IV的体外抗真菌活性,并在小鼠和小鼠体内检查了它们对亚急性系统性念珠菌病的口服药效。豚鼠浅表皮肤癣菌病。化合物2、12、38、39和92表现出较强的口服抗真DOI:10.1021/jm00389a016
-
作为产物:描述:N-甲氧基-n-甲基-2,4-二氯苯甲酰胺 、 正丁基锂 在 盐酸 、 乙酸乙酯 、 Brine 、 Sodium sulfate-III 、 crude product 、 Isohexane ethyl acetate 、 silica 作用下, 以 四氢呋喃 为溶剂, 反应 4.0h, 以to yield 1-(2,4-dichloro-phenyl)-pentan-1-one as a brown oil的产率得到2',4'-二氯苯戊酮参考文献:名称:Organic compounds摘要:这里描述了一些嘧唑并[5.1-b]噁唑衍生物,它们可作为促肾上腺皮质激素释放因子(CRF1)受体拮抗剂。公开号:US07994203B2
文献信息
-
Aerobic Oxidative Dehydrogenation of Ketones to 1,4-Enediones作者:Bao-Yin Zhao、Xing-Long Zhang、Rui-Li Guo、Meng-Yue Wang、Ya-Ru Gao、Yong-Qiang WangDOI:10.1021/acs.orglett.0c04174日期:2021.2.19An efficient and unprecedented strategy for the synthesis of 1,4-enediones from saturated ketones has been developed via palladium-catalyzed oxidative dehydrogenation. The protocol employs molecular oxygen as the sole oxidant and represents an atom- and step-economic process. The approach showed broad substrate scope, good functional group tolerance, and complete E-stereoselectivity. The reaction mechanism
-
Efficient use of trimethylsulfonium methylsulfate as a reagent for the epoxidation of carbonyl-containing compounds作者:Julie Forrester、Ray V. H. Jones、Peter N. Preston、Elizabeth S. C. SimpsonDOI:10.1039/a907053b日期:——A process for the efficient conversion of trimethylsulfonium methylsulfate into dimethylsulfonium methanide, thence epoxides, has been devised. Thus dimethyl sulfate and dimethyl sulfide are caused to react in the presence of either a mineral acid or an organic acid. The anion of trimethylsulfonium methyl sulfate is converted by the acid into methyl hydrogen sulfate, which in turn reacts with excess dimethyl sulfide to form trimethylsulfonium hydrogen sulfate. The outcome is that both methyl groups of dimethyl sulfate are converted into the desired trimethylsulfonium salt, thence dimethylsulfonium methanide; the process is mediated by low boiling dimethyl sulfide which can be recycled, and the by-product (K2SO4) is more easily disposed of than potassium methyl sulfate.
-
Forrester, Julie; Jones, Ray V. H; Preston, Peter N., Journal of the Chemical Society. Perkin transactions I, 1993, # 17, p. 1937 - 1938作者:Forrester, Julie、Jones, Ray V. H、Preston, Peter N.、Simpson, Elisabeth S. C.DOI:——日期:——
-
Synthesis and Biological Activity Evaluation of 1,2,3-Thiadiazole Derivatives as Potential Elicitors with Highly Systemic Acquired Resistance作者:Zhijin Fan、Zugui Shi、Haike Zhang、Xiufeng Liu、Lili Bao、Lin Ma、Xiang Zuo、Qinxiang Zheng、Na MiDOI:10.1021/jf8031364日期:2009.5.27Elicitors provide a broad spectrum of systemic acquired resistance by altering the physical and physiological status of the host plants and, therefore, are among the most successful directions in modern pesticide development for plant protection. To develop a novel elicitor with highly systemic acquired resistance, two series of thiazole- and oxadiazole-containing thiadiazole derivatives were rationally designed and synthesized according to the principle of combination of bioactive substructures in this work. Their structures were characterized by (1)H nuclear magnetic resonance (NMR), infrared (IR), high-resolution mass spectrometry (HRMS), or elemental analysis. Their potential systemic acquired resistance as an elicitor was also evaluated; bioassay results indicated that, among the 23 compounds synthesized, three compounds, 10a, 10d, and 12b, displayed better systemic acquired resistance than the positive control, tiadinil, a commercialized 1,2,3-thiadiazole-based elicitor. In addition, three other compounds, 10f, 12c, and 12j, exhibited a certain degree of fungus growth inhibition in vitro or in vivo. Our results demonstrated that, in combination of bioactive substructures is an interesting exploration for novel pesticide development, thiazole- and oxadiazole-containing thiadiazole derivatives are potential elicitors with good systemic acquired resistance.
-
Forrester, Julie; Jones, Ray V. H.; Preston, Peter N., Journal of the Chemical Society. Perkin transactions I, 1995, # 18, p. 2289 - 2292作者:Forrester, Julie、Jones, Ray V. H.、Preston, Peter N.、Simpson, Elizabeth S. C.DOI:——日期:——
表征谱图
-
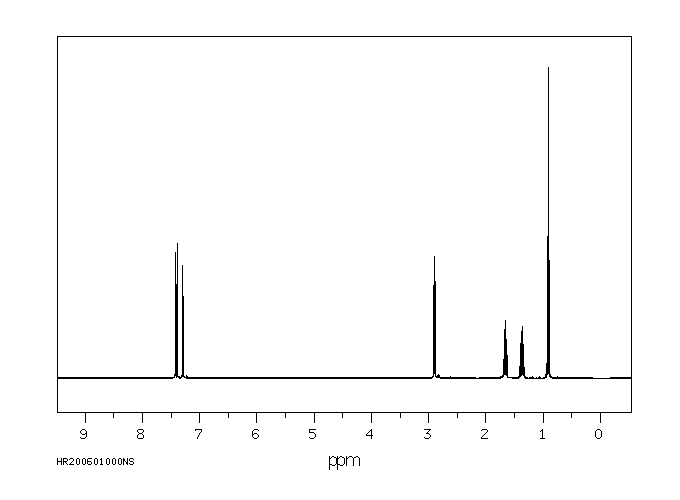
氢谱1HNMR
-
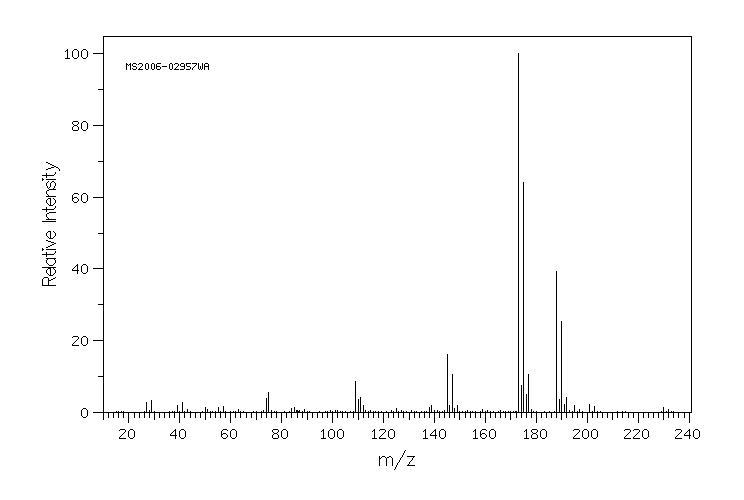
质谱MS
-
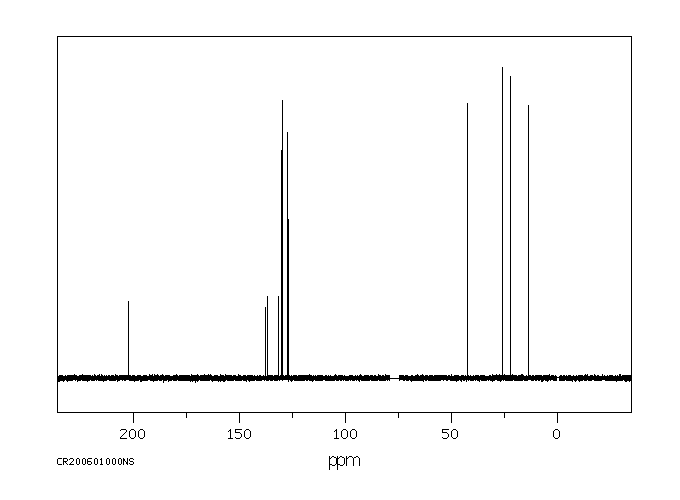
碳谱13CNMR
-
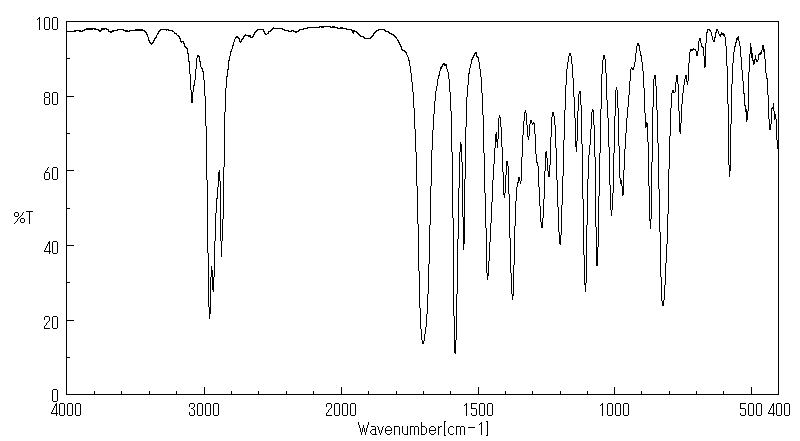
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷