2,2':5',2'-四噻吩-5-甲醛 | 7342-41-8
中文名称
2,2':5',2'-四噻吩-5-甲醛
中文别名
2,2‘:5',2”-三噻吩-5-甲醛;2,2‘:5’,2”-三噻吩-5-甲醛;2,2‘:5",2”-三噻吩-5-甲醛
英文名称
2,2':5',2''-terthiophene-5-carboxaldehyde
英文别名
2,2':5',2''-terthiophene-5-carbaldehyde;5-formyl-2,2':5',2''-terthiophene;[2,2′:5′,2″-terthiophene]-5-carbaldehyde;ecliptal;2-formyl-alpha-terthiophene;5-[5-(2-Thienyl)-2-thienyl]thiophene-2-carbaldehyde;5-(5-thiophen-2-ylthiophen-2-yl)thiophene-2-carbaldehyde
CAS
7342-41-8
化学式
C13H8OS3
mdl
MFCD00115181
分子量
276.404
InChiKey
PMPDDPJYARBNGV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133 °C
-
沸点:435.1±45.0 °C(Predicted)
-
密度:1.381±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:17
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:102
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2934999090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H317,H319
-
储存条件:密封储存于阴凉干燥处。
SDS
2,2':5',2''-Terthiophene-5-carboxaldehyde
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2':5',2''-Terthiophene-5-carboxaldehyde
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2':5',2''-Terthiophene-5-carboxaldehyde
Percent: >98.0%(LC)
CAS Number: 7342-41-8
Synonyms: 5-Formyl-2,2':5',2''-terthiophene
Chemical Formula: C13H8OS3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2,2':5',2''-Terthiophene-5-carboxaldehyde
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Pale yellow - Reddish yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:141°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents]
2,2':5',2''-Terthiophene-5-carboxaldehyde
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Soluble: Hot toluene
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,2':5',2''-Terthiophene-5-carboxaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2':5',2''-Terthiophene-5-carboxaldehyde
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2':5',2''-Terthiophene-5-carboxaldehyde
Percent: >98.0%(LC)
CAS Number: 7342-41-8
Synonyms: 5-Formyl-2,2':5',2''-terthiophene
Chemical Formula: C13H8OS3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2,2':5',2''-Terthiophene-5-carboxaldehyde
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Pale yellow - Reddish yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:141°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents]
2,2':5',2''-Terthiophene-5-carboxaldehyde
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Soluble: Hot toluene
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,2':5',2''-Terthiophene-5-carboxaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,2-联噻吩-5-乙醛 2,2'-bithiophene-5-carboxaldehyde 3779-27-9 C9H6OS2 194.278 alpha-三联噻吩甲醇 α-terthienylmethanol 13059-93-3 C13H10OS3 278.42 alpha-三联噻吩 2,2':5',2''-terthiophene 1081-34-1 C12H8S3 248.394 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-methyl-2,2':5',2''-terthiophene 26905-73-7 C13H10S3 262.42 5-(5-噻吩-2-基噻吩-2-基)噻吩-2-羧酸 [2,2':5',2''-terthiophene]-5-carboxylic acid 87145-85-5 C13H8O2S3 292.403 5-溴-5'-甲酰基-2,2':5'2'-三噻吩 5''-bromo-[2,2':5',2''-terthiophene]-5-carbaldehyde 161726-69-8 C13H7BrOS3 355.3 alpha-三联噻吩甲醇 α-terthienylmethanol 13059-93-3 C13H10OS3 278.42 —— 5-ethynyl-2,2':5',2''-terthiophene 87337-92-6 C14H8S3 272.416 —— Methyl-[2,2';5',2'']terthiophen-5-ylmethyl-amine 174563-37-2 C14H13NS3 291.462 —— 5-difluoromethyl-2,2':5',2"-terthiophene 138691-18-6 C13H8F2S3 298.401 —— (5-(5-(thiophen-2-yl)thiophen-2-yl)thiophen-2-yl)propylamine 866455-92-7 C15H15NS3 305.489 —— 3-[2,2';5',2'']Terthiophen-5-yl-propionitrile 761457-78-7 C15H11NS3 301.457 —— 1,1-dibromo-2-(2,2':5',2''-terthienyl-5-yl)ethylene 87350-66-1 C14H8Br2S3 432.224 —— (E)-bis(2,2':5',2''-terthiophene-5-yl)ethylene 107267-11-8 C26H16S6 520.809 —— 5''-(4-(diphenylamino)phenyl)-2,2';5',2''-terthiophen-5-yl-carbaldehyde 1017260-46-6 C31H21NOS3 519.712 —— 5-heptadecyl-2,2':5',2''-terthiophene 162896-35-7 C29H42S3 486.85 —— 5-acetoxymethyl-2,2':5',2''-terthiophene 26905-77-1 C15H12O2S3 320.457 —— 5-bromo-5''-hydroxymethyl-2,2':5',2''-terthiophene 162896-44-8 C13H9BrOS3 357.316 —— (E)-3-(2,2':5',2"-terthien-5-yl)propenoic Acid 137584-58-8 C15H10O2S3 318.441 —— 2,2':5',2''-terthiophene-5-(3'''-propionic acid) 134273-59-9 C15H12O2S3 320.457 —— methyl (E)-3-(2,2':5',2''-terthien-5-yl)propenoate 134273-57-7 C16H12O2S3 332.468 —— 5-isobutyryloxymethyl-[2,2';5',2'']terthiophene 26905-75-9 C17H16O2S3 348.511 —— methyl 2,2':5',2''-terthiophene-5-(3'''-propionate) 134273-58-8 C16H14O2S3 334.484 α-七噻吩 2,2':5',2'':5'',2''':5''',2'''':5'''',2''''':5''''',2''''''-septithiophene 86100-63-2 C28H16S7 576.897 —— 5-(3-methyl-2-butenoyloxy)methyl-2,2':5',2''-terthiophene 26905-76-0 C18H16O2S3 360.522 —— 5-bromo-5''-heptadecyl-2,2':5',2''-terthiophene 162896-40-4 C29H41BrS3 565.747 —— [5-(5-thiophen-2-ylthiophen-2-yl)thiophen-2-yl]methyl (E)-2-methylbut-2-enoate 26901-19-9 C18H16O2S3 360.522 —— 1-[5-(5-thiophen-2-ylthiophen-2-yl)thiophen-2-yl]-N-[(1R,2R)-2-[[5-(5-thiophen-2-ylthiophen-2-yl)thiophen-2-yl]methylideneamino]cyclohexyl]methanimine 916526-82-4 C32H26N2S6 630.968 —— 5-(1-hydroxyheptadecyl)-2,2':5',2''-terthiophene 162896-38-0 C29H42OS3 502.85 —— (1R,2R)-N,N'-bis-[2,2';5',2'']terthiophen-5-ylmethyl-cyclohexane-1,2-diamine 910643-16-2 C32H30N2S6 635.0 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:The synthesis and photoactivated cytotoxicity of novel 5-phenyl-3-(2,2′:5′,2″-terthien-5-yl)-4,5-dihydro-1H-pyrazolines摘要:A series of novel 5-pheny1-3-(2,2':5',2 ''-terthien-5-yl)-4,5-dihydro-1H-pyrazolines were synthesized in this report. Their photoactivated cytotoxicities on the Spodoptera litura (SL) cell line were evaluated using the MTT method. It was noticed that the inhibitory activities of all the conjugates was enhanced when irradiated with UV-A light. Compounds 4,6 and 8 were found to be most effective with inhibition rates of 88.1%, 88.0%, and 90.5%, respectively. For compound 5, the inhibition rate value was only slightly enhanced under the irradiation treatment (78.3%) compared to the dark treatment (74.8%). The relationship analysis between structure and activity showed that the middle thiophene ring played an important role on the inhibitory activities. It was shown that these compounds could be the potential candidates for new photoactivated pesticides. (C) 2013 Zhuo-Hong Yang. Published by Elsevier B.V. on behalf of Chinese Chemical Society. All rights reserved.DOI:10.1016/j.cclet.2013.11.007
-
作为产物:描述:2,2'-联二噻吩 在 (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride potassium fluoride 、 N-碘代丁二酰亚胺 作用下, 以 甲醇 、 N,N-二甲基甲酰胺 、 甲苯 为溶剂, 反应 0.17h, 生成 2,2':5',2'-四噻吩-5-甲醛参考文献:名称:溶液相微波辅助合成未取代和修饰的α-奎宁和六噻吩摘要:描述了在液相微波辐射下容易合成难溶的未取代和修饰的α-喹啉和六噻吩的方法。微波辐射的使用使得这些化合物可通过铃木交叉偶联反应在几分钟内以高收率制备。通过一锅法的硼化/ Suzuki反应在10分钟内获得未取代的六噻吩,根据非常简单的程序进行纯化,并以84%的产率分离。报道了显示液晶性质的两种新的甲基化喹啉和六噻吩的有效合成。还描述了一种新的微波辅助方法,该方法可将醛基封端的喹啉和六噻吩转化为相应的氰基衍生物。微波的使用扩展到了Sonogashira偶联反应,发现在制备含炔间隔基的喹噻吩中非常有效。已报道并讨论了该化合物的电子和光学特性,以及未取代的喹噻吩的特性。DOI:10.1021/jo035723q
文献信息
-
[EN] IMPROVED OLIGOTHIOPHENES<br/>[FR] OLIGOTHIOPHÈNES AMÉLIORÉS申请人:COMMW SCIENT IND RES ORG公开号:WO2011137487A1公开(公告)日:2011-11-10Compounds of Formula (I), wherein T is independently selected from the group consisting of (a). And the variables R1, R2, R3, R4, R5, R6, R7, R8, R9, Ar, L and n are as defined in the specification herein. The compounds are capable of charge transportation and have application in organic photovoltaic devices such as dye sensitised solar cells.式(I)的化合物,其中T是从(a)组成的群体中独立选择的。变量R1、R2、R3、R4、R5、R6、R7、R8、R9、Ar、L和n的定义如本说明书中所述。这些化合物能够进行电荷传输,并在有机光伏器件中应用,例如染料敏化太阳能电池。
-
Synthesis and photophysical properties of benzo[<i>c</i>]thiophene, <i>p</i>-phenylene-, triphenylamine-, and pyrene-based vinylenes作者:Meganathan Nandakumar、Elumalai Sankar、Arasambattu K. MohanakrishnanDOI:10.1080/00397911.2016.1228111日期:2016.11.16ABSTRACT An efficient synthesis of benzo[c]thiophenyl/p-phenylenyl/pyrenyl phosphonate esters has been achieved using ZnBr2-catalyzed Michaelis–Arbuzov reaction of corresponding benzyl alcohol/bromides at room temperature. Horner–Wadsworth–Emmons reaction of the phosphonate esters with aryl/heteroaryl aldehydes in the presence of t-BuOK furnished the vinylenes in good yields. The absorption and emission
-
Novel Chiral Diamino-Oligothiophenes as Valuable Ligands in Pd-Catalyzed Allylic Alkylations. On the “Primary” Role of “Secondary” Interactions in Asymmetric Catalysis作者:Vincenzo Giulio Albano、Marco Bandini、Manuela Melucci、Magda Monari、Fabio Piccinelli、Simona Tommasi、Achille Umani-RonchiDOI:10.1002/adsc.200505109日期:2005.10A new class of chiral C2-symmetrical diamino-oligothiophenes is described to be effective in catalyzing Pd-mediated asymmetric allylic alkylations in a highly enantioselective manner. The combination of experimental as well as crystallographic evidence revealed the key role played by sulfur-based heteroaromatic rings in the stereodiscriminating step of the procedure. In particular, unprecedented non-covalent
-
New chiral diamino-bis(tert-thiophene): an effective ligand for Pd- and Zn-catalyzed asymmetric transformations作者:Marco Bandini、Manuela Melucci、Fabio Piccinelli、Riccardo Sinisi、Simona Tommasi、Achille Umani-RonchiDOI:10.1039/b711666g日期:——Enantiomerically pure diamino-bis(tert-thiophene) 1b proved to be a valuable and flexible chiral ligand for Pd- and Zn-catalyzed transformations, allowing for high levels of stereocontrol in asymmetric allylic alkylation (ee up to 99%) and hydrosilylations of prochiral carbonyls (ee up to 97%).
-
FULLERENE DERIVATIVE, AND METHOD OF PREPARING THE SAME
表征谱图
-
氢谱1HNMR
-
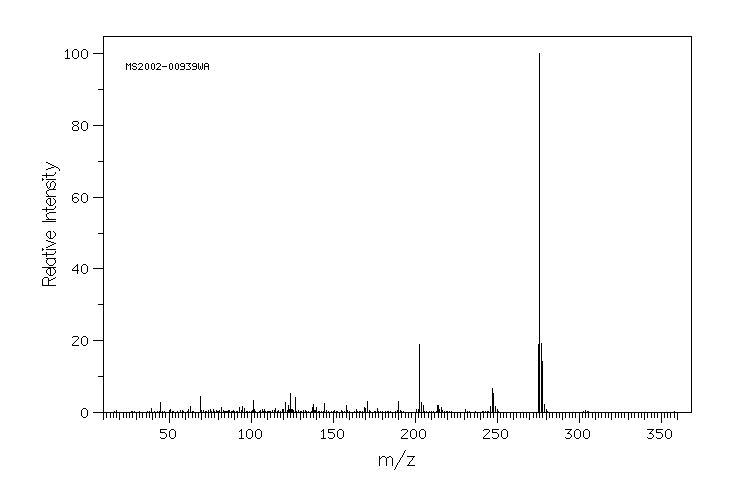
质谱MS
-
碳谱13CNMR
-
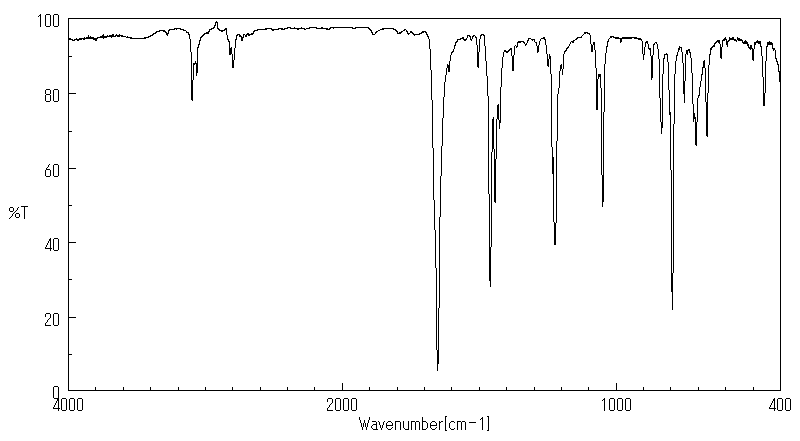
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锡烷,1,1'-(3,3'-二烷基[2,2'-二噻吩]-5,5'-二基)双[1,1,1-三甲基-
试剂5,10-Bis((5-octylthiophen-2-yl)dithieno[2,3-d:2',3'-d']benzo[1,2-b:4,5-b']dithiophene-2,7-diyl)bis(trimethylstannane)
试剂2,2'-Thieno[3,2-b]thiophene-2,5-diylbis-3-thiophenecarboxylicacid
试剂1,1'-[4,8-Bis[5-(dodecylthio)-2-thienyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并[b]噻吩,3-(2-噻嗯基)-
聚(3-己基噻吩-2,5-二基)(区域规则)
甲基[2,3'-联噻吩]-5-羧酸甲酯
牛蒡子醇 B
噻吩并[3,4-B]吡嗪,5,7-二-2-噻吩-
噻吩[3,4-B]吡嗪,5,7-双(5-溴-2-噻吩)-
十四氟-Alpha-六噻吩
三丁基(5''-己基-[2,2':5',2''-三联噻吩]-5-基)锡
α-四联噻吩
α-六噻吩
α-五联噻吩
α-七噻吩
α,ω-二己基四噻吩 5,5′-双(3-己基-2-噻吩基)-2,2′-联噻吩
α,ω-二己基六联噻吩
Α-八噻吩
alpha-三联噻吩甲醇
alpha-三联噻吩
[3,3-Bi噻吩]-2,2-二羧醛
[2,2’]-双噻吩-5,5‘-二甲醛
[2,2':5',2''-三联噻吩]-5,5''-二基双[三甲基硅烷]
[2,2'-联噻吩]-5-甲醇,5'-(1-丙炔-1-基)-
[2,2'-联噻吩]-5-甲酸甲酯
[2,2'-联噻吩]-5-乙酸,a-羟基-5'-(1-炔丙基)-(9CI)
IN1538,4,6-双(4-癸基噻吩基)-噻吩并[3,4-C][1,2,5]噻二唑(S)
C-[2,2-二硫代苯-5-基甲基]胺
6,6,12,12-四(4-己基苯基)-6,12-二氢二噻吩并[2,3-D:2',3'-D']-S-苯并二茚并[1,2-B:5,6-B']二噻吩-2,8-双三甲基锡
5’-己基-2,2’-联噻吩-5-硼酸频哪醇酯
5-辛基-1,3-二(噻吩-2-基)-4H-噻吩并[3,4-c]吡咯-4,6(5H)-二酮
5-苯基-2,2'-联噻吩
5-溴5'-辛基-2,2'-联噻吩
5-溴-5′-己基-2,2′-联噻吩
5-溴-5'-甲酰基-2,2':5'2'-三噻吩
5-溴-3,3'-二己基-2,2'-联噻吩
5-溴-3'-癸基-2,2':5',2''-三联噻吩
5-溴-2,2-双噻吩
5-溴-2,2'-联噻吩-5'-甲醛
5-氯-5'-苯基-2,2'-联噻吩
5-氯-2,2'-联噻吩
5-正辛基-2,2'-并噻吩
5-己基-5'-乙烯基-2,2'-联噻吩
5-己基-2,2-二噻吩
5-全氟己基-5'-溴-2,2'-二噻吩
5-全氟己基-2,2′-联噻吩
5-乙酰基-2,2-噻吩基
5-乙氧基-2,2'-联噻吩
5-丙酰基-2,2-二噻吩