2,4,6-三异丙基-1,3,5-三氧杂环己烷 | 7580-12-3
中文名称
2,4,6-三异丙基-1,3,5-三氧杂环己烷
中文别名
——
英文名称
2,4,6-triisopropyl-1,3,5-trioxane
英文别名
2,4,6-tris(1-methylethyl)-1,3,5-trioxane;2,4,6-tris-isopropyl-1,3,5-trioxane;triisopropyl-s-trioxane;2,4,6-triisopropyl-[1,3,5]trioxane;2,4,6-Triisopropyl-[1,3,5]trioxan;2,4,6-Triisopropyl-(1,3,5)-trioxane;2,4,6-tri(propan-2-yl)-1,3,5-trioxane
CAS
7580-12-3
化学式
C12H24O3
mdl
——
分子量
216.321
InChiKey
XYIANCZIPATGDH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64℃
-
沸点:244.9±20.0 °C(Predicted)
-
密度:0.916±0.06 g/cm3(Predicted)
-
保留指数:1230;1160
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
SDS
反应信息
-
作为反应物:描述:2,4,6-三异丙基-1,3,5-三氧杂环己烷 生成 alkaline earth salt of/the/ methylsulfuric acid参考文献:名称:Barbaglia, Gazzetta Chimica Italiana, 1886, vol. 16, p. 435摘要:DOI:
-
作为产物:描述:特戊醛 在 [P(2-pyridyl)3W(CO)(NO)2](2+)(BF4(1-))2 作用下, 反应 24.0h, 以86%的产率得到2,4,6-三异丙基-1,3,5-三氧杂环己烷参考文献:名称:Tandem reactions of Friedel–Crafts/aldehyde cyclotrimerization catalyzed by an organotungsten Lewis acid摘要:The tris(2-pyridyl)phosphine complex [P(2-py)(3)W(CO)(NO)(2)](BF4)(2) acts as a Lewis acid catalyst precursor for the tandem reactions of Friedel-Crafts/aldehyde cyclotrimerization, which lead to the formation of a series of hyper-branched star polymers. (C) 2002 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(01)02323-1
文献信息
-
[EN] NITRILE COMPOUND AND ITS USE IN PEST CONTROL<br/>[FR] COMPOSE NITRILE ET SON UTILISATION POUR LE CONTROLE DES INSECTES ET ANIMAUX NUISIBLES申请人:SUMITOMO CHEMICAL CO公开号:WO2005063694A1公开(公告)日:2005-07-14The present invention provides a nitrile compound represented by the formula (I): wherein R represents C1-C4 fluoroalkyl, Q represents halogen, C1-C11 alkyl optionally substituted with halogen, C2-C6 alkenyl group optionally substituted with halogen, C2-C6 alkynyl optionally substituted with halogen, C3-C7 cycloalkyl optionally substituted with halogen or (C3-C7 cycloalkyl optionally substituted with halogen)C1-C4 alkyl, which has excellent control effect against pests.本发明提供了一种由式(I)表示的腈化合物:其中R代表C1-C4氟烷基,Q代表卤素,C1-C11烷基可选择地取代卤素,C2-C6烯基基团可选择地取代卤素,C2-C6炔基可选择地取代卤素,C3-C7环烷基可选择地取代卤素或(C3-C7环烷基可选择地取代卤素)C1-C4烷基,对害虫具有出色的控制效果。
-
The reactions of diphenyllithioarsine and diphenylarsine with aldehydes作者:P.J. Busse、C-P. Hrung、K.J. Irgolic、D.H. O'Brien、F.L. Kolar、Omar A. El SeoudDOI:10.1016/s0022-328x(00)94398-5日期:1980.1Diphenyllithioarsine reacts with aldehydes (RCHO, R CH2CH3, CH(CH3)2, and Ph) to form lithium salts of α-hydroxyalkylarsines. Protonation of the lithium salts gives α-hydroxyalkylarsines. Diphenylarsine reacts with aldehydes below room temperature in the absence of solvents to produce white solids. The reactions are rapid in the presence of acid catalysts. Proton and carbon-13 NMR, infrared and Raman
-
Tetranuclear BINOL−Titanium Complex in Selective Direct Aldol Additions作者:Bernd Schetter、Burkhard Ziemer、Gregor Schnakenburg、Rainer MahrwaldDOI:10.1021/jo7014054日期:2008.2.1the direct aldol addition with high degrees of regioselectivity at the sterically more encumbered α-side of unsymmetrical ketones. The formation of quaternary stereocenters is described. Oxygen-containing ene components can also be used as starting material in these reactions. When used with aliphatic aldehydes, acetals 18 or acetals of aldol adducts 20 were observed. As few as 0.2 mol % loadings with
-
Acetonyltriphenylphosphonium bromide in organic synthesis: a useful catalyst in the cyclotrimerization of aldehydes作者:Yung-Son Hon、Chia-Fu LeeDOI:10.1016/s0040-4020(01)00588-9日期:2001.7Acetonyltriphenylphosphonium bromide (ATPB) is a useful catalyst for the cyclotrimerization of the aliphatic aldehydes under solvent-free condition. The aldehydes tethered with a variety of functionality such as olefin, ether, ester, bromide, azide and diester could also be cyclotrimerized under the catalysis of ATPB.
-
Reactions of Trimethylsilyl Isothiocyanate with Aldehydes and Acetals. Synthesis of Symmetrically and Unsymmetrically Isothiocyanato-Substituted Ethers作者:Kozaburo Nishiyama、Makoto ObaDOI:10.1246/bcsj.60.2289日期:1987.6The reactions of trimethylsilyl isothiocyanate (TMSTC) with aldehydes catalyzed by Lewis acid gave α,α′-diisothiocyanato ethers in good to excellent yields. Similarly, unsymmetrical α-isothiocyanato ethers were obtained from the reactions of TMSTC with acetals in appropriate yields.
表征谱图
-
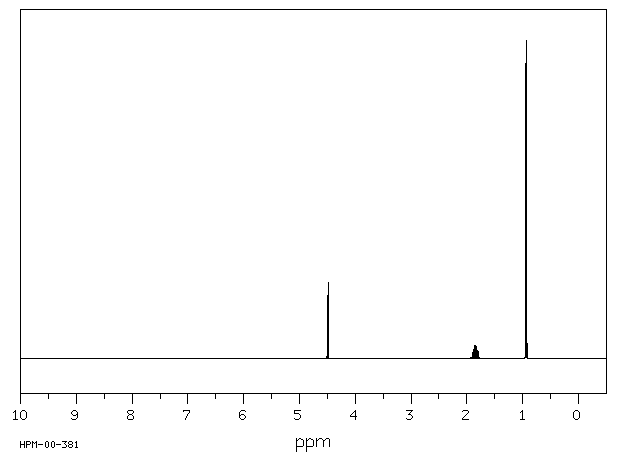
氢谱1HNMR
-
质谱MS
-
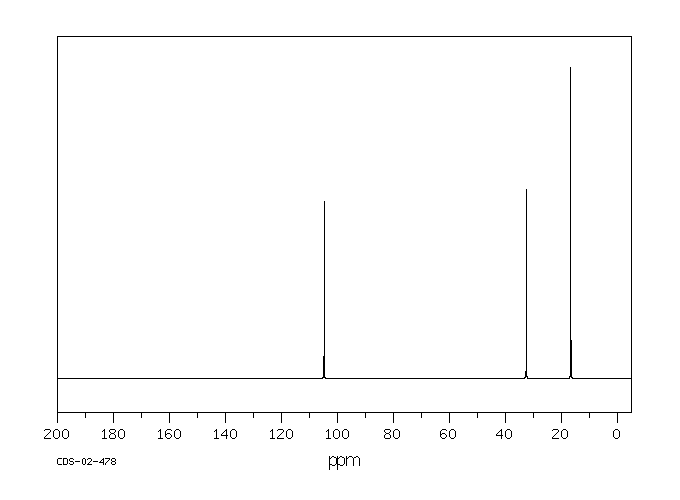
碳谱13CNMR
-
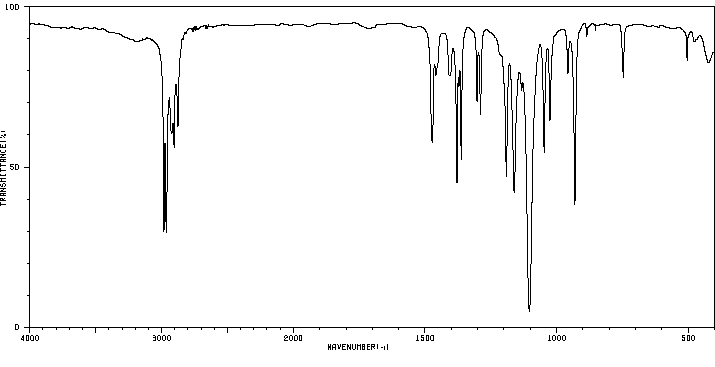
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
氯乙醛三聚物
三聚甲醛
三聚乙醛
S-三噁烷-13C3
9-氟-3,3-二甲基-1,2,5-三氧杂螺[5.5]十一烷-9-羧酸
6-甲基-2,4-二乙基-1,3,5-三恶烷
6,7a-二苯基螺[4a,7a-二氢-4aH-环戊二烯并[2,1-e]1,2,4-三氧杂环己烷-3,1'-环己烷]-5-酮
4-(1,3,5-三氧杂环己烷-2-基)哒嗪
3-苯基-1,2,5-三氧杂螺[5.5]十一碳-7,10-二烯-9-酮
3,3-二甲基-1,2,5-三氧杂螺[5.5]十一烷-9-羧酸
2-氮杂二环[2.2.1]庚-5-烯-3-羧酸,乙基酯(9CI)
2-乙基-4,6-二甲基-1,3,5-三氧杂环己烷
2,4,6-三异丙基-1,3,5-三氧杂环己烷
2,4,6-三异丁基-1,3,5-三氧杂环己烷
2,4,6-三壬基-1,3,5-三氧杂环己烷
2,4,6-三乙基-1,3,5-三氧杂环己烷
2,4,6-三丙基-1,3,5-三氧杂环己烷
2,4,6-三(苯基甲基)-1,3,5-三氧杂环己烷
2,4,6-三(二氯甲基)-1,3,5-三氧杂环己烷
2,4,6-三(2-氯乙基)-1,3,5-三氧杂环己烷
1,3-二氧杂环庚烷与1,3,5-三氧杂环己烷的聚合物
1,3,5-三氧杂环己烷与环氧乙烷的聚合物
1,3,5-三氧杂环己烷与2,2-1,4-丁烷二基二(氧基亚甲基)二环氧乙烷和1,3-二氧戊环的聚合物
1,3,5-三氧杂环己烷与1,3-二氧戊环的聚合物
(4aS,7aS)-6,7a-二苯基螺[7,4a,7a-三氢环戊二烯并[2,1-e]1,2,4-三氧杂环己烷-3,1'-环戊烷]
(Z)-N,N-diethyl-3-(6'-methylspiro[tricyclo[3.3.1.13,7]decane-2,3'-[1,2,4]trioxan]-6'-yl)acrylamide
4-(1,2,4-Trioxolan-3-yl)-2-butanon
6-tert-butyl-3-methyl-1,2,4-trioxan-5-one
2,4-Dibutyl-6-pentyl-1,3,5-trioxan
5-Adamantylen-1,3-dioxan
2,4,6,8-Tetraaethyl-1,3,5,7-tetraxan
1-[1,3]Dioxetan-2-ylmethyl-cyclohexanecarbonitrile
4r,5c-bis-chloromethyl-2ξ-methyl-[1,3]dioxolane
p-Dioxen-dioxetan
5-(3-[1,3]dioxolan-2-yl-propyl)-1-methyl-2-(2-methyl-[1,3]dioxolan-2-yl)-9-aza-bicyclo[3.3.1]nonane
(2-Isopropyl-[1,3]dioxan-5-yl)-dimethyl-sulfonium
2-(4-Methyl-pentyl)-[1,3]dioxetane
(Z)-N,N-diethyl-3-(8-methyl-6,7,10-trioxa-spiro[4.5]dec-8-yl)-acrylamide
β-Cyano-propionaldehyd-trimer, 2.4.6-Tris-2-cyano-aethyl-1.3.5-trioxan
(1,3,5-trioxane-2,4,6-triyl)trimethanamine
(3S,5R)-5-methoxy-3-methylspiro[1,2,4-trioxane-6,2'-adamantane]
12,12-Dimethyl-2,4,8,10-tetraoxa-tricyclo[4.4.4.01,6]tetradecane
1,6-Dichlor-1,6-dideoxy-2,4:3,5-di-O-methylen-L-idit
α-Multistriatin-d3
6-deoxy-2,4:3,5-di-O-methylene-L-gulitol
δ-Multistriatin-4,11,11-d3