α-chloropropionaldehyde trimer | 142817-71-8
中文名称
——
中文别名
——
英文名称
α-chloropropionaldehyde trimer
英文别名
2,4,6-tris(1-chloroethyl)-1,3,5-trioxane
CAS
142817-71-8
化学式
C9H15Cl3O3
mdl
MFCD01632187
分子量
277.575
InChiKey
XTQUGQZRFTWKFM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:96°C
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:α-chloropropionaldehyde trimer 在 Montmorillonite K10 作用下, 反应 0.5h, 生成 2-氯丙醛参考文献:名称:One-pot Preparation of 2-Chloromethyldioxolanes and 2-Aminothiazoles from Chloromethyltrioxanes摘要:在蒙脱石粘土的催化剂量存在下,氯甲基三氧烷的热降解生成了高纯度的α-氯醛,这些产物分别与乙二醇或硫脲在原位处理后,分别得到了2-氯甲基二氧戊环和2-氨基噻唑。所用的粘土催化剂通过过滤被去除。DOI:10.1246/cl.1994.2039
文献信息
-
Stabilization of reactive aldehydes by complexation with methylaluminum bis(2,6-diphenylphenoxide) and their synthetic application作者:Keiji Maruoka、Arnel B. Concepcion、Noriaki Murase、Masataka Oishi、Naoki Hirayama、Hisashi YamamotoDOI:10.1021/ja00063a013日期:1993.5Reactive aldehydes such as formaldehyde and α-chloro aldehydes can be successfully generated by treatment of readily available trioxane and α-chloro aldehyde trimers, respectively, with methylaluminum bis(2,6-diphenylphenoxide) (MAPH), and stabilized as their 1:1 coordination complexes with MAPH. The resulting CH 2 =O.MAPH complex reacts with a variety of olefins to furnish ene-reaction products with
-
CONDENSED BENZAMIDE COMPOUNDS AND INHIBITORS OF VANILLOID RECEPTOR SUBTYPE 1 (VR1) ACTIVITY申请人:Koga Yoshihisa公开号:US20060035882A1公开(公告)日:2006-02-16To provide a compound having an excellent inhibitory effect on vanilloid receptor subtype 1 (VR1) activity which is effective in treating diseases to which the vanilloid receptor subtype 1 (VR1) activity is involved, such as pain, acute pain, chronic pain, neuropathic pain, rheumatoid arthritis pain, neuralgia, etc. and a pharmaceutical composition containing the compound. The object has been attained by a condensed benzamide compound represented by the following formula (the symbols in the formula have the same meanings defined in the specification) or its salt:提供一种对vanilloid受体亚型1(VR1)活性具有出色抑制作用的化合物,该化合物对于治疗与vanilloid受体亚型1(VR1)活性有关的疾病具有有效性,如疼痛、急性疼痛、慢性疼痛、神经病性疼痛、类风湿性关节炎疼痛、神经痛等,以及含有该化合物的药物组合物。该目标通过下面的式子所代表的缩合苯甲酰胺化合物(式子中的符号在说明书中定义了相同的含义)或其盐实现:
-
FUSED BENZAMIDE COMPOUND AND VANILLOID RECEPTOR 1 (VR1) ACTIVITY INHIBITOR申请人:Japan Tobacco, Inc.公开号:EP1777225A1公开(公告)日:2007-04-25To provide a compound having an excellent inhibitory effect on vanilloid receptor subtype 1 (VR1) activity which is effective in treating diseases to which the vanilloid receptor subtype 1 (VR1) activity is involved, such as pain, acute pain, chronic pain, neuropathic pain, rheumatoid arthritis pain, neuralgia, etc. and a pharmaceutical composition containing the compound. The object has been attained by a condensed benzamide compound represented by the following formula (the symbols in the formula have the same meanings defined in the specification) or its salt:提供一种对香草素受体亚型 1(VR1)活性具有优异抑制作用的化合物,该化合物可有效治疗与香草素受体亚型 1(VR1)活性有关的疾病,如疼痛、急性疼痛、慢性疼痛、神经病理性疼痛、类风湿性关节炎疼痛、神经痛等,以及含有该化合物的药物组合物。由下式(式中符号与说明书中定义的含义相同)代表的缩合苯甲酰胺化合物或其盐已达到上述目的:
-
Condensed benzamide compounds as inhibitors of vanilloid receptor subtype 1 (VR1) activity申请人:Japan Tobacco, Inc.公开号:EP2314585A1公开(公告)日:2011-04-27To provide a compound having an excellent inhibitory effect on vanilloid receptor subtype 1 (VR1) activity which is effective in treating diseases to which the vanilloid receptor subtype 1 (VR1) activity is involved, such as pain, acute pain, chronic pain, neuropathic pain, rheumatoid arthritis pain, neuralgia, etc. and a pharmaceutical composition containing the compound. The object has been attained by a condensed benzamide compound represented by the following formula (the symbols in the formula have the same meanings defined in the specification) or its salt:提供一种对香草素受体亚型 1(VR1)活性具有优异抑制作用的化合物,该化合物可有效治疗与香草素受体亚型 1(VR1)活性有关的疾病,如疼痛、急性疼痛、慢性疼痛、神经病理性疼痛、类风湿性关节炎疼痛、神经痛等,以及含有该化合物的药物组合物。由下式(式中符号与说明书中定义的含义相同)代表的缩合苯甲酰胺化合物或其盐已达到上述目的:
-
Wakasugi Takashi, Miyakawa Tadashi, Suzuki Fukuichi, Itsuno Shinichi, Ito+, Chem. Lett, (1994) N 11, S 2039-2042作者:Wakasugi Takashi, Miyakawa Tadashi, Suzuki Fukuichi, Itsuno Shinichi, Ito+DOI:——日期:——
表征谱图
-
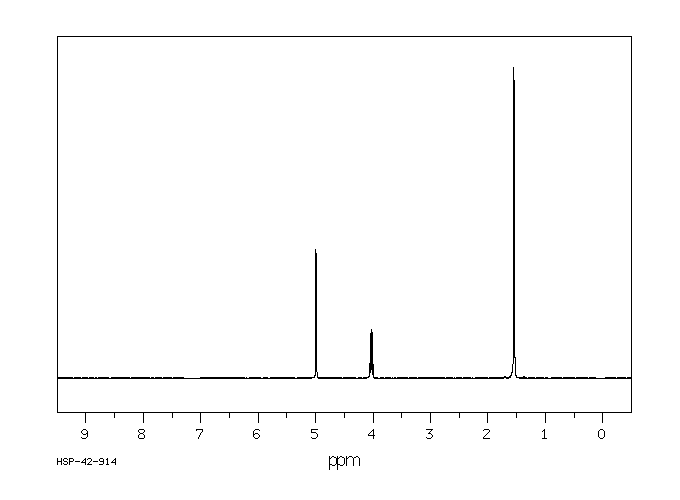
氢谱1HNMR
-
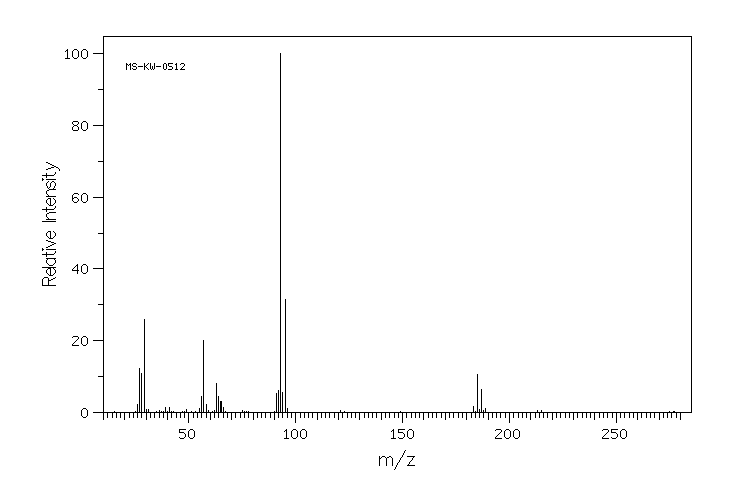
质谱MS
-
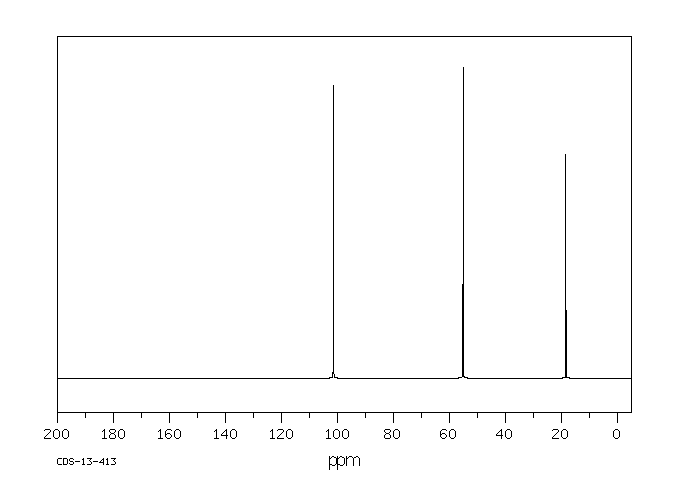
碳谱13CNMR
-
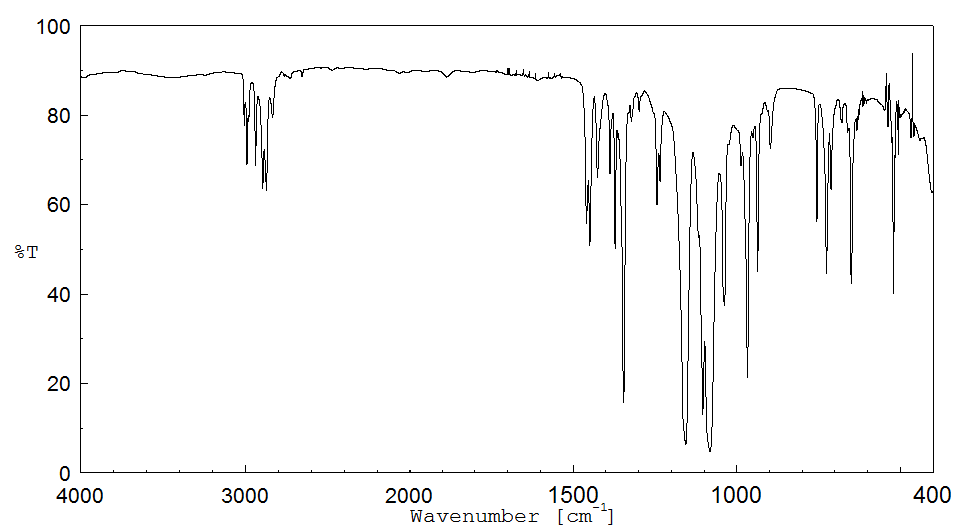
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
氯乙醛三聚物
三聚甲醛
三聚乙醛
S-三噁烷-13C3
9-氟-3,3-二甲基-1,2,5-三氧杂螺[5.5]十一烷-9-羧酸
6-甲基-2,4-二乙基-1,3,5-三恶烷
6,7a-二苯基螺[4a,7a-二氢-4aH-环戊二烯并[2,1-e]1,2,4-三氧杂环己烷-3,1'-环己烷]-5-酮
4-(1,3,5-三氧杂环己烷-2-基)哒嗪
3-苯基-1,2,5-三氧杂螺[5.5]十一碳-7,10-二烯-9-酮
3,3-二甲基-1,2,5-三氧杂螺[5.5]十一烷-9-羧酸
2-氮杂二环[2.2.1]庚-5-烯-3-羧酸,乙基酯(9CI)
2-乙基-4,6-二甲基-1,3,5-三氧杂环己烷
2,4,6-三异丙基-1,3,5-三氧杂环己烷
2,4,6-三异丁基-1,3,5-三氧杂环己烷
2,4,6-三壬基-1,3,5-三氧杂环己烷
2,4,6-三乙基-1,3,5-三氧杂环己烷
2,4,6-三丙基-1,3,5-三氧杂环己烷
2,4,6-三(苯基甲基)-1,3,5-三氧杂环己烷
2,4,6-三(二氯甲基)-1,3,5-三氧杂环己烷
2,4,6-三(2-氯乙基)-1,3,5-三氧杂环己烷
1,3-二氧杂环庚烷与1,3,5-三氧杂环己烷的聚合物
1,3,5-三氧杂环己烷与环氧乙烷的聚合物
1,3,5-三氧杂环己烷与2,2-1,4-丁烷二基二(氧基亚甲基)二环氧乙烷和1,3-二氧戊环的聚合物
1,3,5-三氧杂环己烷与1,3-二氧戊环的聚合物
(4aS,7aS)-6,7a-二苯基螺[7,4a,7a-三氢环戊二烯并[2,1-e]1,2,4-三氧杂环己烷-3,1'-环戊烷]
(Z)-N,N-diethyl-3-(6'-methylspiro[tricyclo[3.3.1.13,7]decane-2,3'-[1,2,4]trioxan]-6'-yl)acrylamide
4-(1,2,4-Trioxolan-3-yl)-2-butanon
6-tert-butyl-3-methyl-1,2,4-trioxan-5-one
2,4-Dibutyl-6-pentyl-1,3,5-trioxan
5-Adamantylen-1,3-dioxan
2,4,6,8-Tetraaethyl-1,3,5,7-tetraxan
1-[1,3]Dioxetan-2-ylmethyl-cyclohexanecarbonitrile
4r,5c-bis-chloromethyl-2ξ-methyl-[1,3]dioxolane
p-Dioxen-dioxetan
5-(3-[1,3]dioxolan-2-yl-propyl)-1-methyl-2-(2-methyl-[1,3]dioxolan-2-yl)-9-aza-bicyclo[3.3.1]nonane
(2-Isopropyl-[1,3]dioxan-5-yl)-dimethyl-sulfonium
2-(4-Methyl-pentyl)-[1,3]dioxetane
(Z)-N,N-diethyl-3-(8-methyl-6,7,10-trioxa-spiro[4.5]dec-8-yl)-acrylamide
β-Cyano-propionaldehyd-trimer, 2.4.6-Tris-2-cyano-aethyl-1.3.5-trioxan
(1,3,5-trioxane-2,4,6-triyl)trimethanamine
(3S,5R)-5-methoxy-3-methylspiro[1,2,4-trioxane-6,2'-adamantane]
12,12-Dimethyl-2,4,8,10-tetraoxa-tricyclo[4.4.4.01,6]tetradecane
1,6-Dichlor-1,6-dideoxy-2,4:3,5-di-O-methylen-L-idit
α-Multistriatin-d3
6-deoxy-2,4:3,5-di-O-methylene-L-gulitol
δ-Multistriatin-4,11,11-d3