2,4,6-三异丙基苯乙酮 | 2234-14-2
中文名称
2,4,6-三异丙基苯乙酮
中文别名
2',4',6'-三异丙基苯乙酮;2’,4’,6’-三异丙基苯乙酮;2",4",6"-三异丙基苯乙酮
英文名称
1-(2,4,6-triisopropylphenyl)ethanone
英文别名
2',4',6'-tri-isopropylacetophenone;1-(2,4,6-triisopropylphenyl)ethan-1-one;2,4,6-Triisopropyl-acetophenon;2',4',6'-Triisopropylacetophenone;1-[2,4,6-tri(propan-2-yl)phenyl]ethanone
CAS
2234-14-2
化学式
C17H26O
mdl
MFCD00015032
分子量
246.393
InChiKey
SGRDDUKVLKWIBZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:84-88 °C
-
沸点:349.39°C (rough estimate)
-
密度:0.9384 (rough estimate)
-
稳定性/保质期:
避免让氧化物直接接触。
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:18
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.588
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
海关编码:2914399090
-
储存条件:将容器密封后,放入一个紧密封装的容器中,并储存在阴凉、干燥的地方。
SDS
| Name: | 2 4 6 -Triisopropylacetophenone 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 2234-14-2 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2234-14-2 | 2',4',6'-Triisopropylacetophenone | 98% | 218-779-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2234-14-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 85 - 87 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C17H26O
Molecular Weight: 246.4
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation.
Incompatibilities with Other Materials:
Oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2234-14-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2',4',6'-Triisopropylacetophenone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 2234-14-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2234-14-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2234-14-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4,6-三异丙基苯甲酸 2,4,6-triisopropylbenzoic acid 49623-71-4 C16H24O2 248.365 2,4,6-三异丙基苯甲酰氯 2,4,6-triisopropylbenzoyl chloride 57199-00-5 C16H23ClO 266.811 1,2,4,5-四异丙苯 1,2,4,5-tetraisopropylbenzene 635-11-0 C18H30 246.436 —— 1-(2,4,6-triisopropylphenyl)ethanol —— C17H28O 248.409 —— 2,4,6-triisopropylphenylacetylene 94804-13-4 C17H24 228.378 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-1-(2,4,6-triisopropylphenyl)ethanone 24153-69-3 C17H25BrO 325.289 —— (E)-3-phenyl-1-(2,4,6-triisopropylphenyl)prop-2-en-1-one 862510-37-0 C24H30O 334.502 —— 1-ethyl-2,4,6-tri-isopropylbenzene 81667-55-2 C17H28 232.409 —— (Z)-2-hydroxy-4-oxo-4-(2,4,6-triisopropylphenyl)but-2-enoic acid —— C19H26O4 318.413 (R)-(+)-1-(2,4,6-三异丙基苯基)乙醇 (R)-1-(2,4,6-triisopropylphenyl)ethanol 181531-14-6 C17H28O 248.409 (S)-(-)-1-(2,4,6-三异丙基苯基)乙醇 (S)-1-(2,4,6-triisopropylphenyl)ethanol 102225-88-7 C17H28O 248.409 —— 1-(2,4,6-triisopropylphenyl)ethanol —— C17H28O 248.409 —— 2,4,6-tri-isopropylstyrene 2039-92-1 C17H26 230.393
反应信息
-
作为反应物:描述:2,4,6-三异丙基苯乙酮 在 4-二甲氨基吡啶 、 氢氧化钾 、 lithium aluminium tetrahydride 作用下, 以 吡啶 、 甲醇 、 乙醚 为溶剂, 反应 6.5h, 生成 (S)-(-)-1-(2,4,6-三异丙基苯基)乙醇参考文献:名称:高效,大规模制备(R)-和(S)-1-(2,4,6-三异丙基苯基)乙醇,用于环戊烯酮,γ-丁内酯和γ-丁内酰胺合成的通用手性助剂摘要:已经开发了一种特别有效,低成本的制备(R)-和(S)-三异丙基苯基)-乙醇的方法,在二氯乙烯-烯醇醚环加成反应中有用的手性控制剂。DOI:10.1016/0957-4166(96)00348-5
-
作为产物:描述:参考文献:名称:13C NMR 研究:第三部分。取代苯乙酮的碳 13 核磁共振谱摘要:已经检查了 55 种取代苯乙酮的 15.1 Mc/s 13C nmr 光谱,并确定了各种碳核的化学位移。该系列包括各种单一替代品...DOI:10.1139/v65-064
文献信息
-
Hydrogen borrowing catalysis using 1° and 2° alcohols: Investigation and scope leading to α and β branched products作者:James R. Frost、Choon Boon Cheong、Wasim M. Akhtar、Dimitri F.J. Caputo、Kirsten E. Christensen、Neil G. Stevenson、Timothy J. DonohoeDOI:10.1016/j.tet.2021.132051日期:2021.4variety of ketones using 1° or 2° alcohols under hydrogen borrowing catalysis is described. Initial research focused on the α-alkylation of cyclopropyl ketones with higher 1° alcohols (i.e. larger than MeOH), leading to the formation of α-branched products. Our search for additional substrates with which to explore this chemistry led us to discover that di-ortho-substituted aryl ketones were also privileged
-
Modulators Of HEC1 Activity And Methods Therefor申请人:Lau Johnson公开号:US20110230486A1公开(公告)日:2011-09-22Compounds, compositions, and methods for modulation of Hec1/Nek2 interaction are provided. Especially preferred compounds disrupt Nek2/Hec1 binding and are therefore useful as chemotherapeutic agent for neoplastic diseases.提供了用于调节Hec1/Nek2相互作用的化合物、组合物和方法。特别偏爱的化合物会破坏Nek2/Hec1的结合,因此可用作肿瘤疾病的化疗药物。
-
[EN] IMPROVED MODULATORS OF HEC1 ACTIVITY AND METHODS THEREFOR<br/>[FR] MODULATEURS AMÉLIORÉS DE L'ACTIVITÉ HEC1 ET PROCÉDÉS ASSOCIÉS申请人:TAIVEX THERAPEUTICS CORP公开号:WO2013082324A1公开(公告)日:2013-06-06Compounds, compositions, and methods for modulation of Hec1/Nek2 interaction are provided. Such compounds disrupt Nek2/Hec1 binding and may be useful as chemotherapeutic agents for neoplastic diseases.提供了用于调节Hec1/Nek2相互作用的化合物、组合物和方法。这些化合物破坏了Nek2/Hec1的结合,并可能作为抗肿瘤疾病的化疗药物有用。
-
Bispentiptycenyl-N-Heterocyclic Carbene (NHC) Gold Complexes: Highly Active Catalysts for the Room Temperature Hydration of Alkynes作者:Maximillian Heidrich、Marvin Bergmann、Dorian Müller-Borges、Herbert PlenioDOI:10.1002/adsc.201800605日期:2018.9.17The virtually quantitative, room temperature hydration of various terminal and internal alkynes in methanol/water requires between 0.01–0.05 mol% of [AuCl(NHC)] activated with 1.5 equiv. of silver triflate and 45 equiv. of triflic acid (both relative to gold complex) with ton of up to 300.000. Iptycenyl‐substituted NHC ligands play the key role and the most efficient NHC ligand is characterized by
-
Iron-Catalyzed α-Alkylation of Ketones with Secondary Alcohols: Access to β-Disubstituted Carbonyl Compounds作者:Léo Bettoni、Sylvain Gaillard、Jean-Luc RenaudDOI:10.1021/acs.orglett.0c00549日期:2020.3.6borrowing hydrogen strategy has been applied in the synthesis of β-branched carbonyl compounds. Various secondary benzylic and aliphatic alcohols have been used as alkylating reagents under mild reaction conditions. The ketones have been isolated in good to excellent yield. Deuterium labeling experiments provide evidence that the alcohol is the hydride source in this reaction and that no reversible step or
表征谱图
-
氢谱1HNMR
-
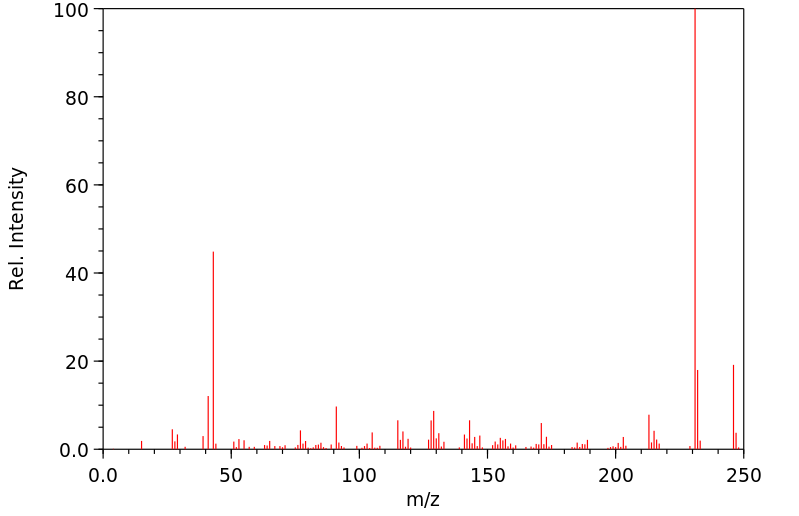
质谱MS
-
碳谱13CNMR
-
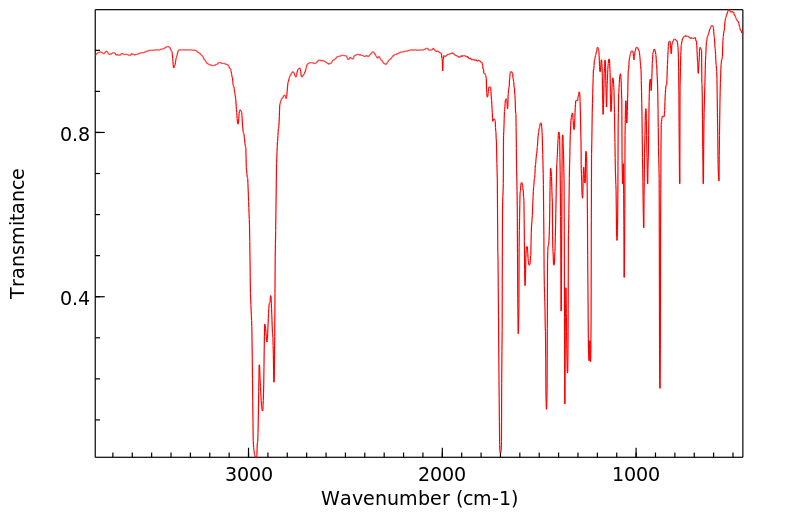
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷