三氧化铬 | 1333-82-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:196 °C (dec.)(lit.)
-
沸点:330 °C
-
密度:2.7
-
闪点:250°C
-
溶解度:1.667g/l
-
暴露限值:a/nm
-
物理描述:Chromium trioxide, anhydrous appears as a dark purplish red solid. Under prolonged exposure to fire or heat the containers may explode. Highly toxic. A confirmed human carcinogen.
-
颜色/状态:Dark red, bipyramidal prismatic crystals, flakes or granular powder
-
气味:Odorless
-
蒸汽压力:Very low (NIOSH, 2016)
-
稳定性/保质期:
CrO3是一种橘红色化合物,受热会逐渐失去氧,最终成为Cr2O3。在较高氧压下,经X射线分析,分解的中间产物还包括Cr3O8、Cr2O5和CrO2。 熔点为196℃,具有剧毒,容易潮解,相对密度为2.7,在高温下易分解,并能溶于水、乙醇、醚以及硫酸和硝酸中,还能与多种有机化合物生成加合物,如 ·2L(其中L可以是吡啶、皮考啉、二甲基吡啶或喹啉等)。不过,它不会与醋酸反应。
遇到臭氧会形成过氧化物,遇过氧化氢则形成过氧化铬酸。当与氯化氢作用时,可生成强氧化剂——氯氧化铬,具有较强的氧化性,与有机物接触摩擦易引发燃烧或爆炸,具备强烈的腐蚀性和毒性。铬化合物对皮肤和黏膜有局部刺激作用,可能导致溃疡。吸入 的气溶胶可造成鼻中隔软骨穿孔,损害呼吸器官,并可能引起肺硬化。一般情况下,会对肝脏、肾脏、胃肠道和心血管系统造成损伤。眼睛接触会导致结膜炎,严重时甚至失明。摄入则会刺激消化道,引发恶心、呕吐、腹痛及血便等症状;重度中毒者会出现呼吸困难、紫绀、休克、肝损害及急性肾功能衰竭等。
较为稳定。应避免与潮湿空气接触。
不会发生聚合反应。
-
自燃温度:May ignite organic materials on contact. (USCG, 1999)
-
分解:Decomposes when heated to 250 °C, liberating oxygen to support combustion.
计算性质
-
辛醇/水分配系数(LogP):-0.36
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.2
-
氢给体数:0
-
氢受体数:3
ADMET
安全信息
-
职业暴露等级:E
-
职业暴露限值:TWA: 0.0002 mg/m3 (8-hours)
-
TSCA:Yes
-
危险等级:5.1
-
立即威胁生命和健康浓度:15 mg Cr(VI)/m3
-
危险品标志:O,T+,N
-
安全说明:S22,S26,S28,S36/37/39,S45,S53,S60,S61
-
危险类别码:R42/43,R9,R24/25,R35,R26,R48/23,R45,R50/53,R62,R46
-
WGK Germany:3
-
海关编码:2819100000
-
危险品运输编号:UN 1463 5.1/PG 2
-
危险类别:5.1
-
RTECS号:GB6650000
-
包装等级:II
-
危险标志:GHS03,GHS05,GHS06,GHS08,GHS09
-
危险性描述:H271,H301 + H311,H314,H317,H330,H334,H340,H350,H361f,H372,H410
-
危险性防范说明:P201,P210,P260,P280,P284,P301 + P310 + P330,P303 + P361 + P353,P304 + P340 + P310,P305 + P351 + P338,P308 + P313,P371 + P380 + P375,P403 + P233
-
储存条件:储存注意事项: - 储存于阴凉、干燥、通风良好的库房。 - 库温不超过30℃,相对湿度不超过80%。 - 包装必须密封,切勿受潮。 - 应与易(可)燃物、还原剂、活性金属粉末、食用化学品分开存放,切忌混储。 - 储区应备有合适的材料收容泄漏物。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | |
| 化学品英文名称: | chromium trioxide |
| 中文名称 2 : | 铬酸酐 |
| 英文名称 2 : | chromic anhydride |
| 技术说明书编码: | 571 |
| CAS No. : | |
| 分子式: | CrO 3 |
| 分子量: | 100.01 |
| 第二部分:成分 / 组成信息 |
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | |
| 健康危害: | |
| 环境危害: | 对环境有危害,对水体可造成污染。 |
| 燃爆危险: | 本品助燃,高毒,为致癌物,具腐蚀性、刺激性,可致人体灼伤。 |
| 第四部分:急救措施 |
| 皮肤接触: | |
| 眼睛接触: | |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 |
| 食入: |
| 第五部分:消防措施 |
| 危险特性: | |
| 有害燃烧产物: | 可能产生有害的毒性烟雾。 |
| 灭火方法: | 采用雾状水、砂土灭火。 |
| 第六部分:泄漏应急处理 |
| 应急处理: |
| 第七部分:操作处置与储存 |
| 操作注意事项: | |
| 储存注意事项: |
| 第八部分:接触控制 / 个体防护 |
| 职业接触限值 | |
| 中国 MAC(mg/m3) : | 0.05[CrO3] |
| 前苏联 MAC(mg/m3) : | 0.01[Cr] |
| TLVTN : | OSHA 0.1mg[CrO3]/m3; ACGIH 0.05mg[Cr]/m3 |
| TLVWN : | 未制定标准 |
| 监测方法: | 二苯碳酰二肼比色法;火焰原子吸收光谱法 |
| 工程控制: | 生产过程密闭,加强通风。提供安全淋浴和洗眼设备。 |
| 呼吸系统防护: | 可能接触其粉尘时,应该佩戴自吸过滤式防尘口罩。必要时,佩戴自给式呼吸器。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿聚乙烯防毒服。 |
| 手防护: | 戴橡胶手套。 |
| 其他防护: | 工作完毕,淋浴更衣。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 主要成分: | 含量 : 工业级 一级≥ 99.5 %。 |
| 外观与性状: | 暗红色或暗紫色斜方结晶,易潮解。 |
| pH : | |
| 熔点 ( ℃ ) : | 196 |
| 沸点 ( ℃ ) : | 分解 |
| 2.70 | |
| 相对蒸气密度 ( 空气 =1) : | 无资料 |
| 饱和蒸气压 (kPa) : | 无资料 |
| 燃烧热 (kJ/mol) : | 无意义 |
| 临界温度 ( ℃ ) : | 无意义 |
| 临界压力 (MPa) : | 无意义 |
| 无资料 | |
| 闪点 ( ℃ ) : | 无意义 |
| 引燃温度 ( ℃ ) : | 无意义 |
| 爆炸上限 %(V/V) : | 无意义 |
| 爆炸下限 %(V/V) : | 无意义 |
| 溶解性: | |
| 主要用途: | 用于电镀工业、医药工业、印刷工业、鞣革和织物媒染。 |
| 其它理化性质: | 230 |
| 第十部分:稳定性和反应活性 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50 : 80 mg/kg( 大鼠经口 ) LC50 :无资料 |
| 亚急性和慢性毒性: | |
| 刺激性: | 高浓度时有明显的局部刺激作用和腐蚀作用。 |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 根据国家和地方有关法规的要求处置。或与厂商或制造商联系,确定处置方法。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| 危险货物编号: | 51519 |
| UN 编号: | 1463 |
| 包装标志: | |
| 包装类别: | O52 |
| 包装方法: | |
| 运输注意事项: |
| 第十五部分:法规信息 |
| 法规信息 |
| 第十六部分:其他信息 |
| 参考文献: | |
| 填表时间: | |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | |
| MSDS 修改日期: | |
制备方法与用途
三氧化铬(化学式:CrO₃)通常呈暗红色斜方晶体,可溶于水、醇、硫酸和乙醚,但不溶于丙酮。与丙酮接触时会发生剧烈爆炸,因此需特别注意安全。容易潮解并在水中生成铬酸。它具有强氧化性。三氧化铬、硫酸及丙酮可用于将醇氧化为羧酸或酮(Jones氧化反应)。此化合物可通过重铬酸钠和浓硫酸的反应制备,并于197 °C以上分解,逐渐失去氧,形成黑色的二氧化铬最终产物为三氧化二铬。它还能与多种有机物如吡啶形成加合物。
危险性急性中毒 吸入三氧化铬气溶胶会引起呼吸道刺激症状、鼻出血、声音嘶哑及鼻粘膜萎缩,严重时可能导致化学性肺炎、呼吸困难、紫绀、休克、肝损害和肾功能衰竭等。口服可刺激消化道,导致恶心、呕吐、腹痛、血便等症状,甚至引发更严重的后果。
慢性影响 长期接触三氧化铬可引起皮肤炎、铬溃疡、鼻炎及呼吸道炎症等。
环境危害 对环境有污染,尤其对水体造成严重破坏。需谨慎处理和储存以防止泄漏。
生产方法 硫酸法将重铬酸钠溶液(70°Bé)与98%的硫酸在反应器中混合加热熔融,至190 °C固体全部熔融后停止加热,物料分层。沉于下层的三氧化铬放入结片机内冷却制得成品。
其他方法其他生产方法包括硝酸法、氟硅酸法和电解法等。其中硫酸法应用最为广泛。
类别与毒性分级- 氧化剂
- 高毒物质
大鼠口服LD₅₀为80毫克/公斤,小鼠为127毫克/公斤。
爆炸物和可燃性危险特性三氧化铬遇还原剂、硫或磷等易爆炸;与可燃物、有机物及还原剂分开存放。
储运特性需在通风、低温、干燥的库房内储存,并远离易燃物品。
灭火方法采用雾状水进行灭火。
职业卫生标准- 时间加权平均容许浓度(TWA):0.05毫克/立方米
- 短时间接触容许浓度(STEL):0.1毫克/立方米
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二氧化铬 chromium dioxide 12018-01-8 CrO2 83.9948
反应信息
-
作为反应物:描述:参考文献:名称:FURUKAWA, SHIGEKI;SUZUKI, YOHICHI, NEHNRE KEKAJSI, 69,(1990) N0, S. 931-939摘要:DOI:
-
作为产物:参考文献:名称:BARROW, PETER;WRIGHT, MICHAEL LAWRENCE摘要:DOI:
-
作为试剂:描述:1-环己烯基-1-甲酸甲酯 在 potassium phosphate 、 copper(l) iodide 、 sodium azide 、 甲烷磺酸 、 palladium 10% on activated carbon 、 (1R,2R)-1,2-diaminocyclohexane 、 水 、 氢气 、 乙酸酐 、 三氧化铬 、 溶剂黄146 、 N,N-二异丙基乙胺 、 N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate 、 sodium hydroxide 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 甲醇 、 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 46.0h, 生成 4-(5-(4-fluoro-1H-indazol-7-yl)-1,2,4-oxadiazol-3-yl)-1-(4-fluorophenyl)-4-methylazepan-2-one参考文献:名称:1,2,4-杂二唑衍生物及其用途摘要:本发明公开了一类1,2,4‑杂二唑衍生物及其用途,属于化学医药技术领域。本发明开发了一类式Ⅰ所示1,2,4‑杂二唑衍生物,其具有激活大麻素受体2的活性,可用于制备CB2小分子激动剂,为治疗包括神经退行性疾病、炎症、疼痛、肝病等在内的多种疾病提供候选药物。#imgabs0#公开号:CN117756793A
文献信息
-
Pyrazoloquinolinone derivatives as protein kinase C inhibitors申请人:——公开号:US20030130277A1公开(公告)日:2003-07-10This invention provides a compound of the formula (I): 1 or the pharmaceutically acceptable salts thereof wherein the dashed lines represent optional double bonds; C 1 , C 2 , C 3 and C 4 are carbon atom; R 1 is C 1-4 alkyl ; R 2 is H, amino, etc.; R 3 is H, halo-CH 2 —, C 2-8 alkyl or Q 1 -, wherein said C 2-8 alkyl is optionally substituted with up to 3 substituents selected from halo, C 1-3 alkyl, R 4 (R 5 )N, etc.; R 4 is H, C 1-7 alkyl, etc.; R 5 is H, C 1-7 alkyl, etc. ; R 6 and R 7 are independently selected from H and C 1-4 alkyl ; R 8 is aryl or heteroaryl ; Q 1 is a 4-12 membered monocyclic or bicyclic aromatic, partially saturated or fully saturated ring optionally containing up to 4 heteroatoms selected from O, N and S, and is optionally substituted with halo, C 1-4 alkyl, etc. ; Y 5 , Y 6 , Y 7 and Y 8 are hydrogen ; Y 1 , Y 2 , Y 3 and Y 4 are independently selected from hydrogen, halo, etc.; Q 2 is a 5-12 membered monocyclic or bicyclic aromatic, partially saturated or fully saturated ring optionally containing up to 3 heteroatoms selected from O, N and S, and is optionally substituted with halo, C 1-4 alkyl-, etc.; These compounds have protein kinase C inhibitory activity and thus are useful for the treatment of neuropathic pain, acute or chronic inflammatory pain, auditory deficiency (synaptic repair), or the like in mammalian, especially humans. This invention also provides a pharmaceutical composition comprising the above compound.这项发明提供了式(I)的化合物: 1 或其药学上可接受的盐,其中虚线代表可选的双键;C 1 、C 2 、C 3 和C 4 是碳原子;R 1 是C 1-4 烷基;R 2 是H、氨基等;R 3 是H、卤代-CH 2 -、C 2-8 烷基或Q 1 -,其中所述的C 2-8 烷基可选地被最多3个从卤素、C 1-3 烷基、R 4 (R 5 )N等中选择的取代基取代;R 4 是H、C 1-7 烷基等;R 5 是H、C 1-7 烷基等;R 6 和R 7 分别选自H和C 1-4 烷基;R 8 是芳基或杂环芳基;Q 1 是一个含有4-12个成员的单环或双环芳香族、部分饱和或完全饱和环,可选地含有最多4个从O、N和S中选择的杂原子,并可选地被卤素、C 1-4 烷基等取代;Y 5 、Y 6 、Y 7 和Y 8 是氢;Y 1 、Y 2 、Y 3 和Y 4 分别选自氢、卤素等;Q 2 是一个含有5-12个成员的单环或双环芳香族、部分饱和或完全饱和环,可选地含有最多3个从O、N和S中选择的杂原子,并可选地被卤素、C 1-4 烷基等取代; 这些化合物具有蛋白激酶C抑制活性,因此对于治疗哺乳动物,特别是人类的神经病痛、急性或慢性炎症性疼痛、听力缺陷(突触修复)等是有用的。该发明还提供了包含上述化合物的药物组合物。
-
Novel compounds申请人:——公开号:US20030144267A1公开(公告)日:2003-07-31The invention provides compounds of general formula (I) wherein Q, R, R 2 , R 4 , R 5 , R 6 , R 7 and R 8 are as defined in the specification, processes for their preparation, pharmaceutical compositions containing them and their use in therapy.这项发明提供了一般式(I)的化合物,其中Q、R、R2、R4、R5、R6、R7和R8如规范中所定义,以及它们的制备方法、含有它们的药物组合物以及它们在治疗中的用途。
-
Kinetics of Oxidation of Allyl Alcohol by Cr(VI)作者:K. G. SEKAR、V. PALANIVELDOI:10.13005/ojc/280254日期:2012.6.18The kinetics of oxidation of allyl alcohol by quinaldinium chlorochromate is investigated in 40 % acetic acid – water (v/v) medium. The order with respect to oxidant and hydrogen ion concentration is found to be one and fractional with respect to substrate. The rate of the reaction is decreases with the decrease in the dielectric constant of the medium. The rate of the reaction has negligible effect
-
Azolobenzazepine derivatives as neurologically active agents申请人:Zeneca Limited公开号:US06124281A1公开(公告)日:2000-09-26The invention relates to azolobenzazepine derivatives of formula (I), wherein: X is O or S; R.sup.1, R.sup.2, R.sup.3 and R.sup.4 are independently hydrogen, perfluorolower-alkyl, halogen, nitro or cyano; and C together with the carbon atoms to which it is attached forms a 5-membered aromatic heterocycle selected from the group consisting of a pyrazol and triazole, to pharmaceutical compositions containing them and to methods for the treatment of neurological disorders utilizing them. ##STR1##
-
Process for the preparation of 6-(perfluoroalkyl) uracil compounds from申请人:American Cyanamid Company公开号:US06140270A1公开(公告)日:2000-10-31An improved process for the preparation of 6-(perfluoroalkyl)uracil compounds having the structural formula I ##STR1## from 2-(N,N-disubstituted)amino-4-(perfluoroalkyl)-1,3-oxazin-6-one compounds having the structural formula II ##STR2##
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
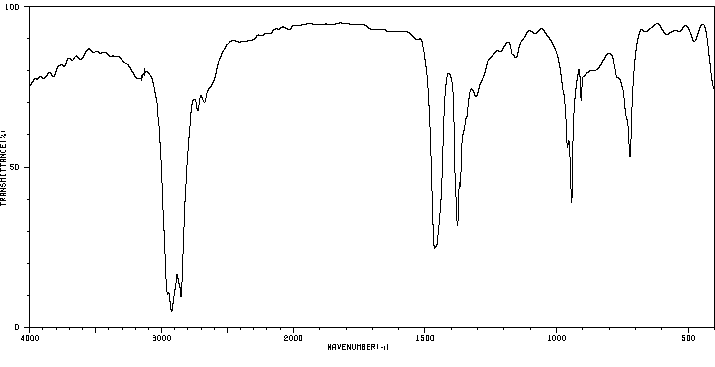
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息