2,7-二甲基菲 | 1576-69-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:101-102 °C
-
沸点:369.6±12.0 °C(Predicted)
-
密度:1.084±0.06 g/cm3(Predicted)
-
保留指数:2027.9;338.24;338.49;337.79;339.1;339.1;337.18;337.83;337.68;339.01;338.63;338.4;339.8;339.9;337.7;339.23;339.23
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):5.6
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,7-二甲基-9-菲酚 2,7-dimethyl-9-phenanthrol 698376-80-6 C16H14O 222.287 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,7-二甲酰基菲 2,7-diformylphenanthrene 106093-06-5 C16H10O2 234.254
反应信息
-
作为反应物:参考文献:名称:一种新型化合物及采用该化合物的有机电致发光器件摘要:本发明涉及一种有机电致发光器件,包括第一电极、第二电极和位于所述第一电极和第二电极之间的一层或多层有机层,所述有机层中包含至少一种由下述通式(I)所示的化合物:式(Ⅰ)中,Ar1至Ar4分别独立选自氢、或选自下式(Ⅱ)所示的结构式,且Ar2和Ar3不同时为氢:式(Ⅱ)中:Ra独立选自氢、C1~C10的烷基、卤素、氰基、硝基、C6~C30的取代或未取代的芳基或稠环芳烃基团、C3~C30的取代或未取代的杂芳基或稠杂环芳烃基团,n=0‑4的整数,且当n=2‑4时,Ra相同或不同。R1与R2之间可以互相连接形成环状结构,R1和R2分别独立选自C1~C10的烷基、卤素、氰基、硝基、C6~C30的取代或未取代的芳基或稠环芳烃基团、C3~C30的取代或未取代的杂芳基或稠杂环芳烃基团。公开号:CN109593086A
-
作为产物:描述:2,7-Bis-chlormethyl-9,10-dihydro-phenanthren 在 palladium on activated charcoal 氢气 、 sulfur 作用下, 生成 2,7-二甲基菲参考文献:名称:Voegtle,F.; Staab,A., Chemische Berichte, 1968, vol. 101, p. 2709 - 2716摘要:DOI:
文献信息
-
Electron transfer in bis-porphyrin donor-acceptor compounds with polyphenylene spacers shows a weak distance dependence作者:Anna Helms、David Heiler、George McLendonDOI:10.1021/ja00041a047日期:1992.7containing one, two, or three phenyl bridges. Complete synthetic details are provided. For studies of photochemical electron transfer, mixed metals were incorporated, with zinc in one porphyrin macrocycle and Fe III (bis-imidazole) in the other macrocycle. When photoexcited, an electron is transferred from Zn to Fe III
-
Regiospecific synthesis of alkylphenanthrenes using a combined directed <i>ortho</i> and remote metalation SuzukiMiyaura cross coupling strategy作者:Xiongwei Cai、Stephen Brown、Peter Hodson、Victor SnieckusDOI:10.1139/v03-179日期:2004.2.1
Using a combined directed ortho metalation (DoM) SuzukiMiyaura cross coupling directed remote metalation (DreM) approach, the alkylphenanthrenes (APs) 1-methyl- (5a), 1,7-dimethyl- (5b), 2,7-dimethyl- (5c), 7-ethyl-1-methyl- (15), and 7-tert-butyl-1-methylphenanthrenes (27) have been synthesized in four to seven steps and 21%36% overall yields. In contrast to classical protocols, this method, which may be scaled to gram quantities, provides single isomers of APs in high purity of value as analytical standards for environmental studies. Aminocarbonylation of triflates to N,N-diethylbenzamides (9 [Formula: see text] 10) and anionic Fries rearrangement (23 [Formula: see text] 24) provide other potential links to DoM chemistry.Key words: phenanthrene, directed ortho metalation, SuzukiMiyaura cross coupling, synthesis, polycyclic aromatic hydrocarbon, carbonylation.
利用结合的定向正金属化(DoM)-Suzuki-Miyaura交叉偶联-定向远程金属化(DreM)方法,已合成了烷基菲(APs)1-甲基-(5a)、1,7-二甲基-(5b)、2,7-二甲基-(5c)、7-乙基-1-甲基-(15)和7-叔丁基-1-甲基菲(27),在四到七个步骤中产率为21%-36%。与传统方法相比,这种方法可以扩展到克量级,提供高纯度的APs单异构体,可用作环境研究的分析标准。三氟甲磺酸酯的氨基羰化反应生成N,N-二乙基苯甲酰胺(9至10),以及阴离子Fries重排反应(23至24)提供了DoM化学的其他潜在联系。关键词:菲、定向正金属化、Suzuki-Miyaura交叉偶联、合成、多环芳烃、羰化。 -
Buckybowls: a simple, conceptually new synthesis of C2v-semibuckminsterfullerene (C30H12, [5,5]-fulvalene circulene)作者:Goverdhan Mehta、Gautam PandaDOI:10.1039/a706336i日期:——An extremely simple synthesis of decacyclic, C30H12 ‘buckybowl’ 2 from m-xylene, employing a strategy of wider applicability for the synthesis of diverse curved aromatic surfaces, is described.
-
106. Synthesis of alkylphenanthrenes. Part VI. Attempts to synthesise the hydrocarbon “C<sub>16</sub>H<sub>14</sub>'” derived from strophanthidin作者:Robert Downs Haworth、Cecil Robert Mavin、George SheldrickDOI:10.1039/jr9340000454日期:——
-
Gready, Jill E.; Hata, Kazumi; Sternhell, Sever, Australian Journal of Chemistry, 1990, vol. 43, # 3, p. 593 - 600作者:Gready, Jill E.、Hata, Kazumi、Sternhell, Sever、Tansey, Charles W.DOI:——日期:——
表征谱图
-
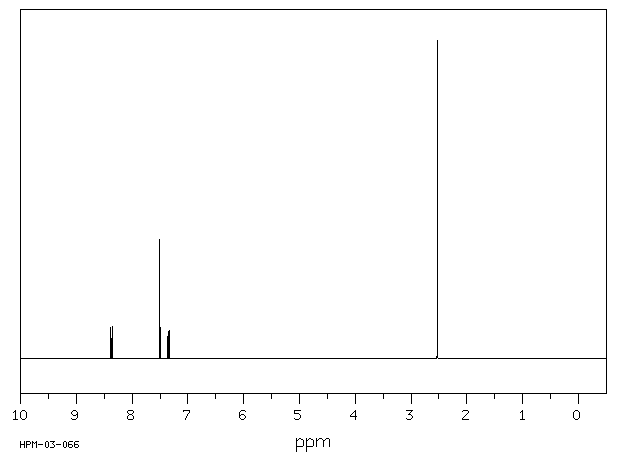
氢谱1HNMR
-
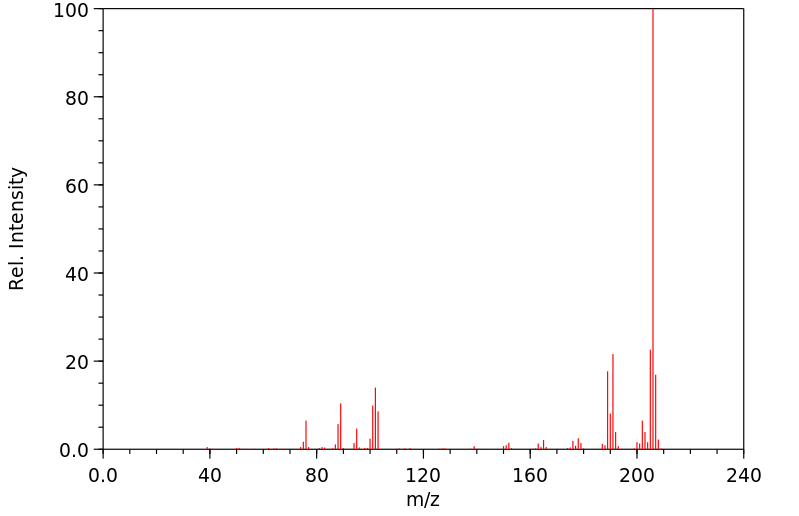
质谱MS
-
碳谱13CNMR
-
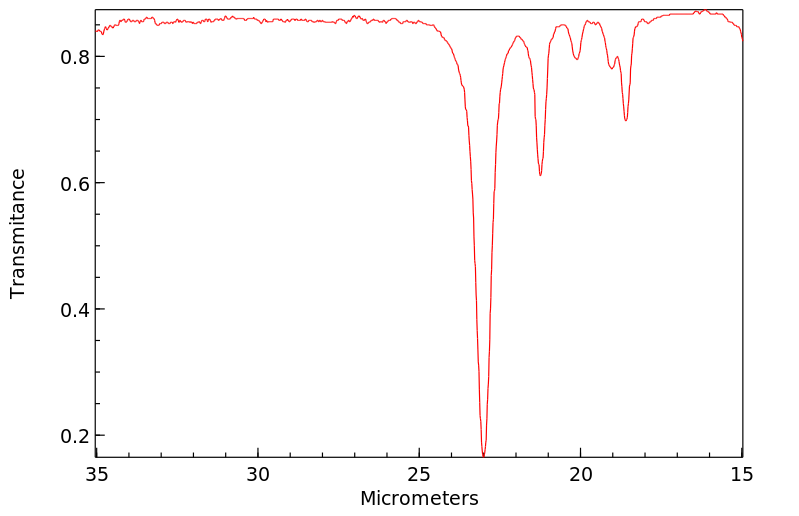
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息