2-(3-甲氧基苯基)-5-苯基-1,3-恶唑 | 38705-20-3
中文名称
2-(3-甲氧基苯基)-5-苯基-1,3-恶唑
中文别名
——
英文名称
2-(3-methoxyphenyl)-5-phenyloxazole
英文别名
2-(3-methoxy-phenyl)-5-phenyl-oxazole;2-(3-Methoxy-phenyl)-5-phenyl-oxazol;5-Phenyl-2-m-methoxyphenyl-oxazol;2-(3-methoxyphenyl)-5-phenyl-1,3-oxazole
CAS
38705-20-3
化学式
C16H13NO2
mdl
——
分子量
251.285
InChiKey
NWCFKCRZJQLJDJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:79-80 °C
-
沸点:429.0±47.0 °C(Predicted)
-
密度:1.142±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:19
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:35.3
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:液体闪烁体。V. 2,5-二芳基恶唑和相关化合物的吸收和荧光光谱1摘要:提供了紫外吸收数据和最大发射波长和荧光平均波长。给出了以前未报道的化合物作为闪烁体溶质的合成和评价。DOI:10.1021/ja01577a032
-
作为产物:描述:3-甲氧基苯甲酰胺 在 三氟甲磺酸三甲基硅酯 、 sodium hydride 、 对甲苯磺酸 、 [双(三氟乙酰氧基)碘]苯 作用下, 以 四氢呋喃 、 乙醚 、 二氯甲烷 、 甲苯 、 mineral oil 为溶剂, 反应 24.0h, 生成 2-(3-甲氧基苯基)-5-苯基-1,3-恶唑参考文献:名称:Iodine(III)-Promoted Synthesis of Oxazoles through Oxidative Cyclization of N-Styrylbenzamides摘要:The hypervalent iodine reagent PhI(OTf)(2), generated in situ, has been successfully utilized in an intramolecular oxidative cyclization of N-styrylbenzamides. In remarkably short reaction times, the desired 2,5-disubstituted oxazoles were isolated in high yields in this metal-free oxidative C-O bond-forming reaction.DOI:10.1055/s-0033-1339491
文献信息
-
Convenient One-Pot Synthesis of 2,5-Disubstituted Oxazoles via a Catalytic Oxidative Dehydrogenation of F<sub>3</sub>CSO<sub>3</sub>H·SiO<sub>2</sub>-DDQ/CuCl<sub>2</sub>/LiCl作者:Shizhen Yuan、Zhen Li、Ling XuDOI:10.1002/jhet.1637日期:2013.11A facile one‐pot synthesis of 2,5‐disubstituted oxazoles was developed via cyclization of aldoximes and phenylacetylene then dehydrogenation oxidation. 2,3‐dichloro‐5,6‐dicyano‐1,4‐benzoquinone was studied for the selective oxidation of oxazolines using Cu2+/Li+ as catalyst and O2 as indirect oxidant. The reaction results showed that this catalyst system can effectively catalyze the oxidation of oxazolines通过醛肟和苯乙炔的环化然后脱氢氧化制得了一种简便的一锅合成2,5-二取代的恶唑。以Cu 2+ / Li +为催化剂,O 2为间接氧化剂,研究了2,3-二氯-5,6-二氰基-1,4-苯醌对氧化唑啉的选择性氧化。反应结果表明,该催化剂体系可有效催化恶唑啉氧化为相应的恶唑。因此,通过2,3-二氯-5,6-二氰基-1,4-苯醌/ CuCl 2 / LiCl / O 2的催化氧化,很容易以高收率合成各种多取代的恶唑。
-
Copper-catalyzed oxidative cyclization of chalcone and benzylic amine leading to 2,5-diaryl oxazoles via carbon–carbon double bond cleavage作者:Dongfang Liu、Jintao Yu、Jiang ChengDOI:10.1016/j.tet.2013.12.077日期:2014.2oxidative cyclization of chalcone with benzylic amine is achieved, providing 2,5-diaryl oxazoles in moderate to good yields. The procedure employs O2 as a clean oxidant and involves an oxidative cleavage of the CC bond as the key step.
-
TBHP/I<sub>2</sub>-Mediated Domino Oxidative Cyclization for One-Pot Synthesis of Polysubstituted Oxazoles作者:Huanfeng Jiang、Huawen Huang、Hua Cao、Chaorong QiDOI:10.1021/ol1023085日期:2010.12.3A facile type of one-pot, transition-metal-free domino process was developed for the synthesis of oxazoles. Thus, a variety of polysubstituted oxazoles were easily synthesized via t-BuOOH/I2-mediated domino oxidative cyclization from readily available starting materials under mild conditions.
-
Nickel-Catalyzed Coupling of Azoles with Aromatic Nitriles作者:Mckenna G. Hanson、Noelle M. Olson、Zubaoyi Yi、Grace Wilson、Dipannita KalyaniDOI:10.1021/acs.orglett.7b01938日期:2017.8.18This manuscript describes the Ni-catalyzed coupling of azoles with aromatic nitriles. The use of BPh3 promotes these arylations with electronically diverse azoles and benzonitriles. While the nickel catalyst is necessary for the arylations of phenyl oxazoles, arylation of benzoxazoles with some nitriles affords the arylated products even in the absence of the Ni catalyst albeit in lower yield than
-
Certain azoles exhibiting ATP-utilizing enzyme inhibitory activity, compositions, and uses thereof申请人:Mendoza Serafin Jose公开号:US20080015193A1公开(公告)日:2008-01-17Certain oxazole-based compounds exhibiting ATP-utilizing enzyme inhibitory activity, methods of using compounds exhibiting ATP-utilizing enzyme inhibitory activity, and compositions comprising compounds exhibiting ATP-utilizing enzyme inhibitory activity, are disclosed.
表征谱图
-
氢谱1HNMR
-
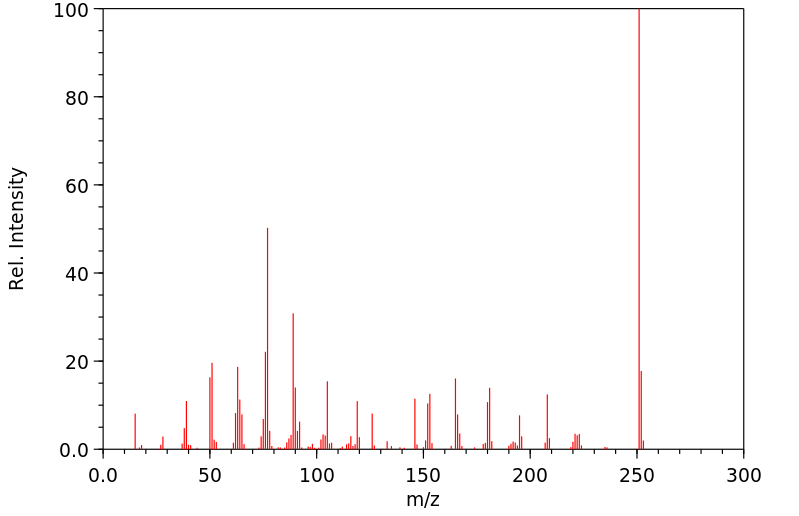
质谱MS
-
碳谱13CNMR
-
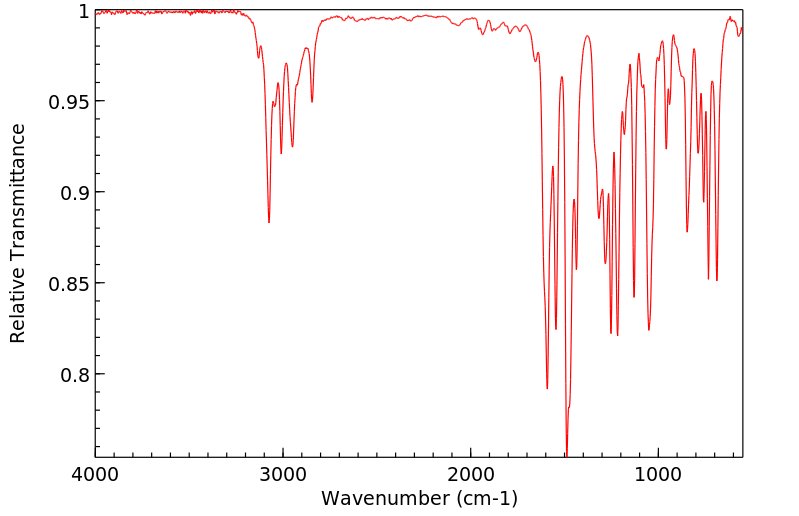
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)