2,2,4-trimethyl-3-oxo-1-pentanol | 15904-30-0
中文名称
——
中文别名
——
英文名称
2,2,4-trimethyl-3-oxo-1-pentanol
英文别名
1-hydroxy-2,2,4-trimethylpentan-3-one;1-hydroxy-2,2,4-trimethyl-3-pentanone;2,2,4-trimethyl-1-hydroxy-3-pentanone;3-Pentanone, 1-hydroxy-2,2,4-trimethyl-
CAS
15904-30-0
化学式
C8H16O2
mdl
——
分子量
144.214
InChiKey
PYSAJDICZJMKGG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:97-98 °C(Press: 20 Torr)
-
密度:0.926±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.88
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methoxy-2,2,4-trimethyl-pentan-3-one 23546-40-9 C9H18O2 158.241
反应信息
-
作为反应物:描述:2,2,4-trimethyl-3-oxo-1-pentanol 在 甲醇 、 (R)-lithium binaphtholate 、 sodium methylate 作用下, 以 四氢呋喃 、 正己烷 为溶剂, 反应 7.34h, 生成 (3S)-2,2,4-trimethyl-pentan-1,3-diol参考文献:名称:Enantioselective Evans-Tishchenko Reduction of b-Hydroxyketone Catalyzed by Lithium Binaphtholate摘要:锂二苯基二萘酚酸盐催化了非对称的埃文斯-季辰科还原反应,将非手性的β-羟基酮转化为单酰保护的1,3-二醇,具有高的立体选择性。在对映体的β-羟基酮反应中,发生了高度立体选择性的动力学光学分辨。DOI:10.3390/molecules16065008
-
作为产物:参考文献:名称:β-羟基酮的非对映选择性形成(通过降低酮二聚体)摘要:开发了通过还原乙烯酮二聚体非对映选择性形成β-羟基酮的一般方法。研究了衍生自烷基芳基烯酮和二甲基烯酮的烯酮均二聚体以及衍生自甲基烯酮和乙基苯基烯酮或二苯基烯酮的烯酮异二聚体的还原。使用LiBH 4以最佳非对映选择性(dr = 6:1)还原甲基苯基乙烯酮二聚体。然而,更一般地说,就非对映选择性(dr高达> 99:1)和产率(10个例子中为62-> 99%)而言,发现LiAlH 4是最有效的还原体系。DOI:10.1016/j.tetlet.2012.12.011
文献信息
-
Chemoselective, iron(ii)-catalyzed oxidation of a variety of secondary alcohols over primary alcohols utilizing H2O2 as the oxidant作者:Matthew Lenze、Eike B. BauerDOI:10.1039/c3cc41131a日期:——A mild, iron-based catalyst system is presented that selectively oxidizes secondary alcohols to the corresponding hydroxy ketones in the presence of primary alcohols within 15 minutes at room temperature, utilizing H2O2 as the oxidant.
-
Hepatitis C Virus Inhibitors申请人:Bristol-Myers Squibb Company公开号:US20130183269A1公开(公告)日:2013-07-18The present disclosure is generally directed to antiviral compounds, and more specifically directed to combinations of compounds which can inhibit the function of the NS5A protein encoded by Hepatitis C virus (HCV), compositions comprising such combinations, and methods for inhibiting the function of the NS5A protein.本公开涉及抗病毒化合物,更具体地涉及能够抑制丙型肝炎病毒(HCV)编码的NS5A蛋白功能的化合物组合,包括这种组合的组成物,以及抑制NS5A蛋白功能的方法。
-
Aerobic Oxidation of Secondary Alcohols with Nitric Acid and Iron(III) Chloride as Catalysts in Fluorinated Alcohol作者:Štefan Možina、Jernej IskraDOI:10.1021/acs.joc.9b02109日期:2019.11.15being weak acceptors. We used 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as the activating solvent for a nitric acid and FeCl3-catalyzed aerobic oxidation of secondary alcohols to ketones. Reaction proceeded selectively with excellent yields with no reaction on the primary alcohol group. Oxidation of benzyl alcohols proceeds selectively to aldehydes with only HNO3 as the catalyst, while reaction on tertiary
-
PYRROLIDINYL SULFONE RORGAMMA MODULATORS申请人:Bristol-Myers Squibb Company公开号:US20150191483A1公开(公告)日:2015-07-09Described are RORγ modulators of the formula (I), or stereoisomers, tautomers, pharmaceutically acceptable salts, solvates, or prodrugs thereof, wherein all substituents are defined herein. Also provided are pharmaceutical compositions comprising the same. Such compounds and compositions are useful in methods for modulating RORγ activity in a cell and methods for treating a subject suffering from a disease or disorder in which the subject would therapeutically benefit from modulation of RORγ activity, for example, autoimmune and/or inflammatory disorders.描述了公式(I)的RORγ调节剂,或其立体异构体、互变异构体、药物可接受的盐、溶剂化物或前药,其中所有取代基都在此定义。还提供了包含相同成分的药物组合物。这类化合物和组合物在调节细胞中RORγ活性的方法和治疗受疾病或紊乱困扰的主体中是有用的,例如,主体将从调节RORγ活性中获益的自免疫和/或炎症紊乱。
-
Manganese-Catalyzed Selective Oxidation of Aliphatic CH groups and Secondary Alcohols to Ketones with Hydrogen Peroxide作者:Jia Jia Dong、Duenpen Unjaroen、Francesco Mecozzi、Emma C. Harvey、Pattama Saisaha、Dirk Pijper、Johannes W. de Boer、Paul Alsters、Ben L. Feringa、Wesley R. BrowneDOI:10.1002/cssc.201300378日期:2013.9An efficient and simple method for selective oxidation of secondary alcohols and oxidation of alkanes to ketones is reported. An in situ prepared catalyst is employed based on manganese(II) salts, pyridine‐2‐carboxylic acid, and butanedione, which provides good‐to‐excellent conversions and yields with high turnover numbers (up to 10 000) with H2O2 as oxidant at ambient temperatures. In substrates bearing
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
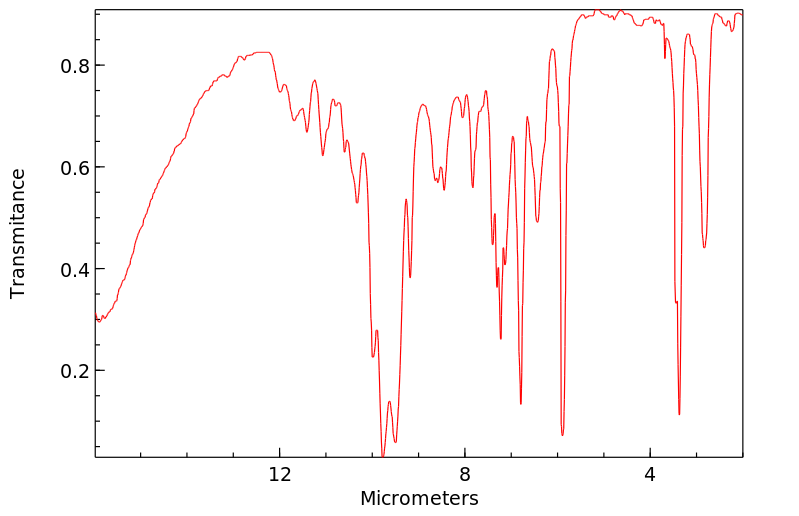
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷