(3E)-4-(2,4,5,6-tetramethylphenyl)-3-buten-2-one | 267901-66-6
中文名称
——
中文别名
——
英文名称
(3E)-4-(2,4,5,6-tetramethylphenyl)-3-buten-2-one
英文别名
4-<2'.3'.4'.6'-Tetramethyl-phenyl>-but-3-en-2-on;4-(2,3,4,6-Tetramethylphenyl)-3-buten-2-one;(E)-4-(2,3,4,6-tetramethylphenyl)but-3-en-2-one
CAS
267901-66-6
化学式
C14H18O
mdl
——
分子量
202.296
InChiKey
PXZVHZRZXKANST-VOTSOKGWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:15
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
反应信息
-
作为反应物:描述:参考文献:名称:Syntheses of γ-Ionone Analogues. III. Diels-Alder Reaction Involving a Mixture of 3, 4-Dimethyl-1, 3-pentadiene and 2, 3-Dimethyl-1, 3-pentadiene, and Some Derivatives from 2-Benzyloxymethyl-4, 5, 6-trimethylcyclohexa-1, 4-dienylmethanal摘要:DOI:10.1246/bcsj.35.45
-
作为产物:描述:2,3-二甲基-1,3-戊二烯 在 barium dihydroxide 、 sulfur 作用下, 以 水 、 苯 为溶剂, 生成 (3E)-4-(2,4,5,6-tetramethylphenyl)-3-buten-2-one参考文献:名称:Syntheses of γ-Ionone Analogues. III. Diels-Alder Reaction Involving a Mixture of 3, 4-Dimethyl-1, 3-pentadiene and 2, 3-Dimethyl-1, 3-pentadiene, and Some Derivatives from 2-Benzyloxymethyl-4, 5, 6-trimethylcyclohexa-1, 4-dienylmethanal摘要:DOI:10.1246/bcsj.35.45
文献信息
-
Novel Pd(II)- and Pt(II)-Catalyzed Regio- and Stereoselective <i>trans</i>-Hydroarylation of Alkynes by Simple Arenes作者:Chengguo Jia、Wenjun Lu、Juzo Oyamada、Tsugio Kitamura、Kenji Matsuda、Masahiro Irie、Yuzo FujiwaraDOI:10.1021/ja0005845日期:2000.8.1Efficient trans-hydroarylation of alkynes by simple arenes has been realized regio- and stereoselectively at room temperature in the presence of Pd(II) or Pt(II) catalysts and a mixed solvent containing trifluoroacetic acid (TFA). Various arenes undergo trans-hydroarylation selectively across terminal and internal C-C triple bonds-including those conjugated to CHO, COMe, CO2H, and CO2Et groups, affording kinetically controlled cis-arylalkenes predominantly in most cases, especially, in good yields for electron-rich arenes and activated alkynes. The formation of arene/alkyne 1/2 or 2/1 adducts as side products is dependent on the arenes' and alkynes' substituents, which can be suppressed in some cases by changing the catalyst, catalyst concentration, and reaction time. The Pt(II) system, PtCl2/2AgOAc/TFA, shows lower catalytic activity than Pd(OAc)(2)/TFA, but higher selectivity, giving higher yields of adducts at the same conversion. On the basis of several isotope experiments and control reactions, a possible mechanism involving electrophilic metalation of aromatic C-H bonds by in-situ-generated cationic Pd(II) and Pt(II) species leading to intermolecular trans-arylpalladation to alkynes has been discussed.
表征谱图
-
氢谱1HNMR
-
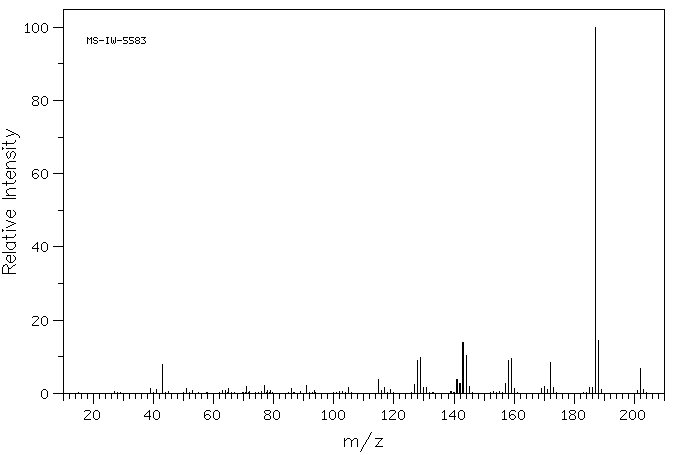
质谱MS
-
碳谱13CNMR
-
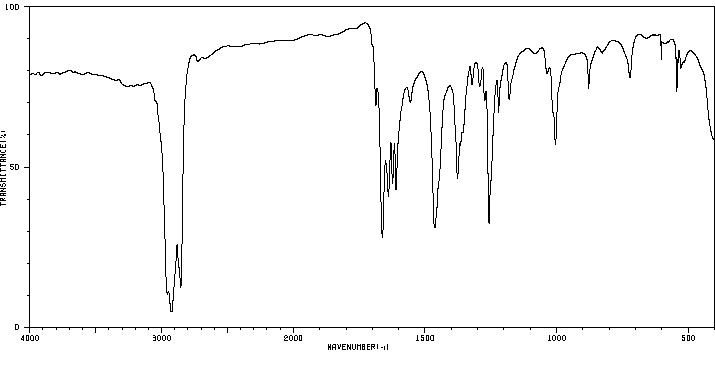
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30