cyclo-octa-1,3,6-triene | 3725-30-2
中文名称
——
中文别名
——
英文名称
cyclo-octa-1,3,6-triene
英文别名
Cyclooctatrien-(1,3,6);Cycloocta-1,3,6-trien;1,3,6-Cyclooctatrien;1,3,6-Cyclooctatriene;(1Z,3Z,6Z)-cycloocta-1,3,6-triene
CAS
3725-30-2
化学式
C8H10
mdl
——
分子量
106.167
InChiKey
LHNSMWDERKGLJK-DKPWQKSPSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-62°C
-
沸点:144.11°C (rough estimate)
-
密度:0.8940
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Sanne,W.; Schlichting,O., Angewandte Chemie, 1963, vol. 75, p. 156 - 161摘要:DOI:
-
作为产物:描述:参考文献:名称:Sanne,W.; Schlichting,O., Angewandte Chemie, 1963, vol. 75, p. 156 - 161摘要:DOI:
文献信息
-
Additionsreaktionen der nitrosogruppe—XV作者:G. Kresze、H. BatheltDOI:10.1016/0040-4020(73)80167-x日期:1973.11,3-cyclooctadiene, 1,3,6-cyclooctatriene and bicyclo-[4.2.0]-2,4-octadiene give normal Diels-Alder-adducts with nitrosobenzenes. In the reaction of 1,3,5-cyclooctatriene with nitro-substituted nitrosobenzenes, too, [2+4]-cycloadducts are formed which, however, rearrange by heating in solution to [2+6]-cycloadducts. These isomers are the only products isolated in the addition of nitrosobenzene and
-
[4 + 2] Cycloaddition reactions of hexachlorotropone作者:M. Akhtar、D.M. Bratby、J.C. Chadwick、G.I. FrayDOI:10.1016/0040-4020(76)85144-7日期:1976.1The cycloaddition of hexachlorotropone to selected olefins, including 1,3-dienes, has been examined. Unlike tropone, which undergoes [6 + 4] cycloadditions with 1,3-dienes, only [4 + 2] processes were observed with hexachlorotropone. Its apparent preference for exo-addition (contrast tetrachlorocyclopentadienone) probably results from thermodynamic control of the endo : exo product ratios.已经研究了六氯四氢环酮与包括1,3-二烯在内的所选烯烃的环加成反应。与托克酮与1,3-二烯经历[6 + 4]环加成反应不同,六氯托酮仅观察到[4 + 2]过程。其表观偏好外-addition(对比度tetrachlorocyclopentadienone)可能导致从热力学控制内:外产物比。
-
The reductive decyclizations of semibullvalene作者:Melvin J. Goldstein、Timothy T. WenzelDOI:10.1039/c39840001654日期:——Reduction of semibullvalene (5) with potassium more closely resembles deprotonation of tetrahydropentalenes by n-butyl-lithium-potassium t-pentoxide than it does the reduction of (5) with lithium; the former prccesses both provide the cyelo-octatetraenyl dianion (4), plausibly via the intermediate bicyclo[3.3.0]octadienediyl dianion (3).
-
Todres, Z. V., Russian Journal of Physical Chemistry, 1980, vol. 54, # 5, p. 631 - 638作者:Todres, Z. V.DOI:——日期:——
-
Echter, Toni; Meier, Herbert, Chemische Berichte, 1985, vol. 118, # 1, p. 182 - 197作者:Echter, Toni、Meier, HerbertDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
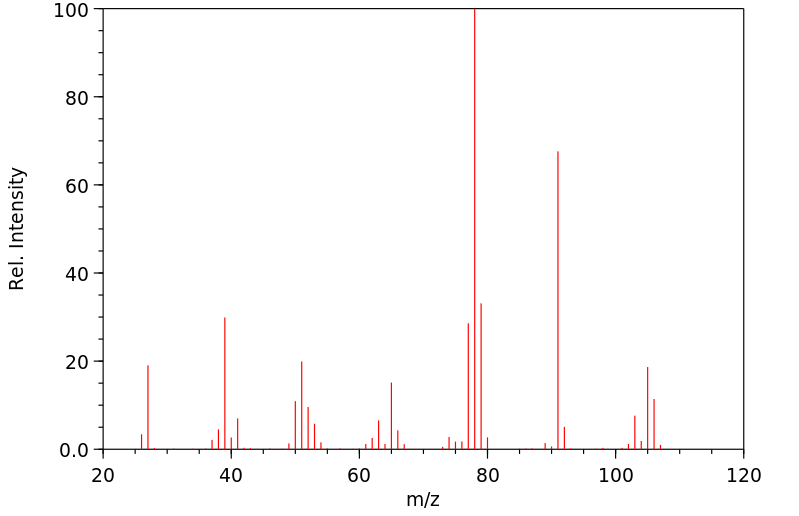
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-