2-丁基-2-辛烯醛 | 99915-14-7
中文名称
2-丁基-2-辛烯醛
中文别名
——
英文名称
(Z)-2-butyloct-2-enal
英文别名
(Z)-2-butyl-2-octenal;2-Butyl-2-octenal
CAS
99915-14-7
化学式
C12H22O
mdl
——
分子量
182.306
InChiKey
LYGMPIZYNJGJKP-BENRWUELSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
LogP:4.785 (est)
-
保留指数:1369;1359;1363;1363;1361
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:13
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2912190090
SDS
反应信息
-
作为反应物:描述:2-丁基-2-辛烯醛 在 哌啶 、 lithium aluminium tetrahydride 作用下, 以 乙醚 为溶剂, 反应 18.0h, 生成 2-Butyl-3-mercapto-octan-1-ol参考文献:名称:2-烷基烷基-2-烯醛和3-(乙酰硫基)-2-烷基烷烃的合成和感官性质。摘要:通过在碱性条件下将硫代乙酸迈克尔加成到α,β-不饱和2-烷基取代的醛上,采用平行合成的方法制备一系列3-(乙酰硫基)-2-烷基链烷醛,这些醛是通过相应的醛醇缩合反应制得的伯醛为原料。根据GC,MS和NMR数据对目标化合物进行了表征。由训练有素的小组来确定气味的感官特性,例如气味质量和气味检测阈值。讨论了结构-活性关系,表明热带/植物味的“嗅觉基团”所需的1,3-氧-硫官能度可进一步扩展至乙酰硫基衍生物。DOI:10.1021/jf0498968
-
作为产物:描述:参考文献:名称:Model Studies on the Pattern of Volatiles Generated in Mixtures of Amino Acids, Lipid-Oxidation-Derived Aldehydes, and Glucose摘要:The development of flavor and browning in thermally treated foods results mainly from the Maillard reaction and lipid degradation but also from the interactions between both reaction pathways. To study these interactions, we analyzed the volatile compounds resulting from model reactions of lysine or glycine with aldehydes originating from lipid oxidation [hexanal, (E)-2-hexenal, or (2E,4E)-decadienal] in the presence and absence of glucose. The main reaction products identified in these model mixtures were carbonyl compounds, resulting essentially from amino-acid-catalyzed aldol condensation reactions. Several 2-alkylfurans were detected as well. Only a few azaheterocyclic compounds were identified, in particular 5-butyl-2-propylpyridine from (E)-2-hexenal model systems and 2-pentylpyridine from (2E,4E)-decadienal model reactions. Although few reaction products were found resulting from the condensation of an amino acid With a lipid-derived aldehyde, the amino acid plays an important role in catalyzing the degradation and further reaction of these carbonyl compounds. These results suggest that amino-acid-induced degradations and further reactions of lipid oxidation products may be of considerable importance in thermally processed foods.DOI:10.1021/jf104091p
文献信息
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
Protection-, Salt-, and Metal-Free Syntheses of [n]-Shogaols by Use of Dimethylammonium Dimethyl Carbamate (DIMCARB) without Protecting Groups作者:Nobuyuki Mase、Kunihiko Takabe、Norihiko KitagawaDOI:10.1055/s-0029-1218389日期:2010.1Shogaols, the pungent principle of ginger, exhibit inter- esting bioactivities. Practical preparation of shogaols is highly de- sired. Here we report the protection/deprotection-, salt-, and metal- free synthesis of shogaol in three steps by use of dimethylammoni- um dimethyl carbamate (DIMCARB), in which DIMCARB smoothly promoted Mannich-type condensation of the ketone do- nor with the aldehyde acceptor
-
[EN] CONVERSION OF ALCOHOLS TO LINEAR AND BRANCHED FUNCTIONALIZED ALKANES<br/>[FR] CONVERSION D'ALCOOLS EN ALCANES FONCTIONNALISÉS LINÉAIRES ET RAMIFIÉS申请人:ORGANOFUEL SWEDEN AB公开号:WO2018034609A1公开(公告)日:2018-02-22Embodiments herein concerns the eco-friendly conversion of simple alcohols to linear or branched functionalized alkanes, by integrated catalysis. The alcohols are firstlyoxidized either chemically or enzymatically to the corresponding aldehydes or ketones, followed by aldol condensations using a catalyst to give the corresponding enals or enones. The enals or enones are subsequently and selectively hydrogenated using a recyclable heterogeneous metal catalyst, organocatalyst or an enzyme to provide linear or branched functionalized alkanes with an aldehyde, keto- or alcohol functionality. The process is also iterative and can be further extended by repeating the above integrated catalysis for producing long-chain functionalized alkanes from simple alcohols.
-
Chloride ion pairs as catalysts for the alkylation of aldehydes and ketones with C–H acidic compounds作者:Camille Carrignon、Philippe Makowski、Markus Antonietti、Frédéric GoettmannDOI:10.1016/j.tetlet.2009.06.013日期:2009.8Chloride anions associated with various soft cations (like tetraalkyl ammoniums, alkyl imidazoliums or pyridiniums) were shown to be able to promote the alkylation of carbonyl derivatives with acidic compounds, as exemplified on Knoevenagel and aldol condensations under relatively mild conditions. This activity was attributed to an enhanced nucleophilicity of the chlorine anion, originating from a
-
COMPOUNDS USEFUL AS MODULATORS OF TRPM8申请人:Senomyx, Inc.公开号:US20170096418A1公开(公告)日:2017-04-06The present disclosure relates to compounds which are useful as cooling sensation compounds.本公开涉及作为冷感化合物有用的化合物。
表征谱图
-
氢谱1HNMR
-
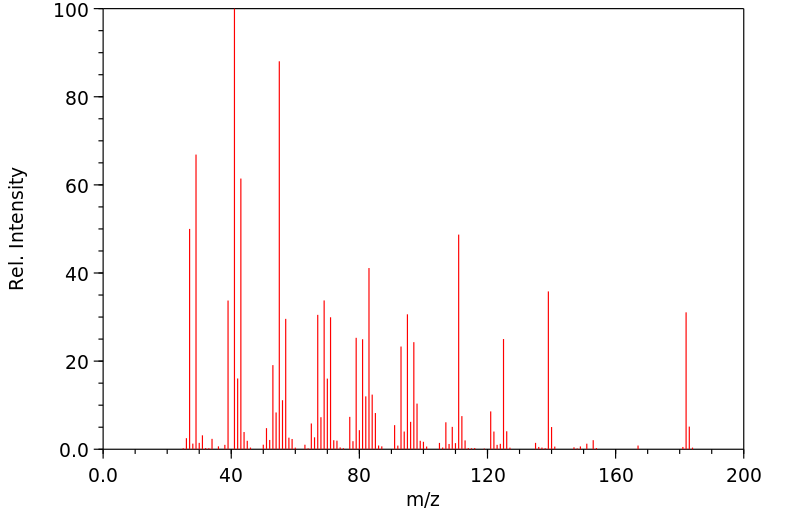
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷