2-氨基喹唑啉 | 1687-51-0
中文名称
2-氨基喹唑啉
中文别名
2-氨基奎唑啉
英文名称
quinazolin-2-ylamine
英文别名
2-aminoquinazoline;2-Amino-chinazolin;quinazolin-2-amine
CAS
1687-51-0
化学式
C8H7N3
mdl
——
分子量
145.164
InChiKey
CZAAKPFIWJXPQT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204 °C
-
沸点:370.7±25.0 °C(Predicted)
-
密度:1.292±0.06 g/cm3(Predicted)
-
保留指数:1748
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Aminoquinazoline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H302: Harmful if swallowed
H319: Causes serious eye irritation
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2-Aminoquinazoline
CAS number: 1687-51-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
Eye contact:
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H7N3
Molecular weight: 145.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Aminoquinazoline
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H302: Harmful if swallowed
H319: Causes serious eye irritation
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 2-Aminoquinazoline
CAS number: 1687-51-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
Eye contact:
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H7N3
Molecular weight: 145.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯喹唑啉 2-chloroquinazoline 6141-13-5 C8H5ClN2 164.594 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-ethylaminoquinazoline 83260-66-6 C10H11N3 173.217 —— N-quinazolin-2-yl-acetamide 103264-36-4 C10H9N3O 187.201 —— N-isopentylquinazolin-2-amine 1380234-91-2 C13H17N3 215.298 —— N-hexylquinazolin-2-amine 1380234-89-8 C14H19N3 229.325 —— N-octylquinazolin-2-amine 1380234-90-1 C16H23N3 257.379 —— N-(2-methylbutyl)quinazolin-2-amine 1421970-35-5 C13H17N3 215.298 —— N-benzylquinazolin-2-amine —— C15H13N3 235.288 —— N-(2-ethylhexyl)quinazolin-2-amine 1380234-92-3 C16H23N3 257.379 —— N-(cyclohexylmethyl)quinazolin-2-amine 1380234-93-4 C15H19N3 241.336 —— N-(4-methylbenzyl)quinazolin-2-amine 1421970-36-6 C16H15N3 249.315 —— N-phenethylquinazolin-2-amine 1335439-90-1 C16H15N3 249.315 —— N-(3-phenylpropyl)quinazolin-2-amine 1380234-94-5 C17H17N3 263.342 —— N-(4-(trifluoromethyl)benzyl)quinazolin-2-amine 1380234-95-6 C16H12F3N3 303.287 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:SASSE K., SYNTHESIS, 1978, NO 5, 379-382摘要:DOI:
-
作为产物:参考文献:名称:硝基,Diradicals和Ylides。2-喹唑基腈的开环扩环和开环摘要:在200°C和更高的温度下升华,将Tetrazolo [1,5- a ]喹唑啉(9)转化为2-azidoquinquinazoline(10),叠氮化物-四唑平衡受熵控制。2-喹唑基氮烯11和27和/或它们的扩环产物14和29可以经历I型(ylidic)和II型(diradicaloidoid)开环。的氩气矩阵光解9 / 10,得到2- quinazolylnitrene(11),它的特点是ESR,UV和IR光谱。还观察到由重排或开环形成的少量第二氮烯。激进主义者(19)通过II型开环快速形成并通过ESR光谱表征; 它在15 K时热衰减,半衰期约为。47分钟,与其计算得出的系统间易穿越性(19T → 19OSS)相一致,然后轻松进行环化/重排成1-氰基吲唑(21)(计算出的活化屏障1-2 kcal / mol)和N-氰基蒽腈(22)。21和22是光解的最终产物。21也是闪蒸真空热解的最终产品。零场分裂参数D(cmDOI:10.1021/jo052541i
-
作为试剂:描述:氯喳味唑酮 、 hydrochloride dihydrate salt 、 ethyl N-(6-amino-2,3-dichlorobenzyl)glycine 、 溴化氰 在 2-氨基喹唑啉 作用下, 以 alcohol 为溶剂, 生成 2-氨基喹唑啉参考文献:名称:Method for the manufacture of Anagrelide摘要:提供了从2,3-二氯苯甲醛制备Anagrelide碱的方法。还提供了一种从2,3-二氯苯甲醛制备中间化合物乙基N-(2,3-二氯-6-硝基苯基)甘氨酸的方法,并使用SnCl2或特定定义的催化剂还原甘氨酸化合物。进一步提供了从相应的亚胺喹唑啉化合物进行环化反应形成Anagrelide碱的方法。公开号:US20030060630A1
文献信息
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
-
Aminoquinazolines and their use as medicaments申请人:——公开号:US20020049197A1公开(公告)日:2002-04-25Compounds of the formula 1 having an inhibitory effect on signal transduction mediated by tyrosine kinases, their use in the treatment of diseases, especially tumoral diseases and diseases of the lungs and air-ways, and the preparation thereof具有抑制酪氨酸激酶介导的信号传导作用的化合物的公式,它们在治疗疾病,特别是肿瘤性疾病和肺部及呼吸道疾病中的用途,以及其制备
-
Aminoquinazolines which inhibit signal transduction mediated by tyrosine kinases申请人:——公开号:US20020082271A1公开(公告)日:2002-06-27Compounds of the formula 1 having an inhibitory effect on signal transduction mediated by tyrosine kinases and their use in the treatment of diseases, especially tumoral diseases and diseases of the lungs and airways, and the preparation thereof.
-
SUBSTITUTED SULFONAMIDE COMPOUNDS申请人:OBERBOERSCH Stefan公开号:US20080153843A1公开(公告)日:2008-06-26Substituted sulfonamide derivatives, a process for their preparation, pharmaceutical compositions containing these compounds, and to the use of substituted sulfonamide derivatives in the treatment or inhibition of pain and/or various disorders or disease states.
-
[DE] HETEROZYKLISCH SUBSTITUIERTE PENTANOL-DERIVATE, VERFAHREN ZU IHRER HERSTELLUNG UND IHRE VERWENDUNG ALS ENTZÜNDUNGSHEMMER<br/>[EN] HETEROCYCLICALLY SUBSTITUTED PENTANOL DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF, AND USE THEREOF AS ANTI-INFLAMMATORY AGENTS<br/>[FR] DERIVES DE PENTANOL SUBSTITUES PAR UN HETEROCYCLE, PROCEDE DE PRODUCTION DE CES COMPOSES ET LEUR UTILISATION COMME AGENTS ANTI-INFLAMMATOIRES申请人:SCHERING AG公开号:WO2005003098A1公开(公告)日:2005-01-13Die Erfindung betrifft durch Chinazolin, Chinoxalin, Cinnolin, Indazol, Phthalazin, Naphthyridin, Benzothiazol, Dihydroindolon, Dihydroisoindolon, Benzimidazol oder Indol substituierte Pentanolderivate der allgemeinen Formel (I) ein Verfahren zu ihrer Herstellung und ihre Verwendung als Entzündungshemmer.
表征谱图
-
氢谱1HNMR
-
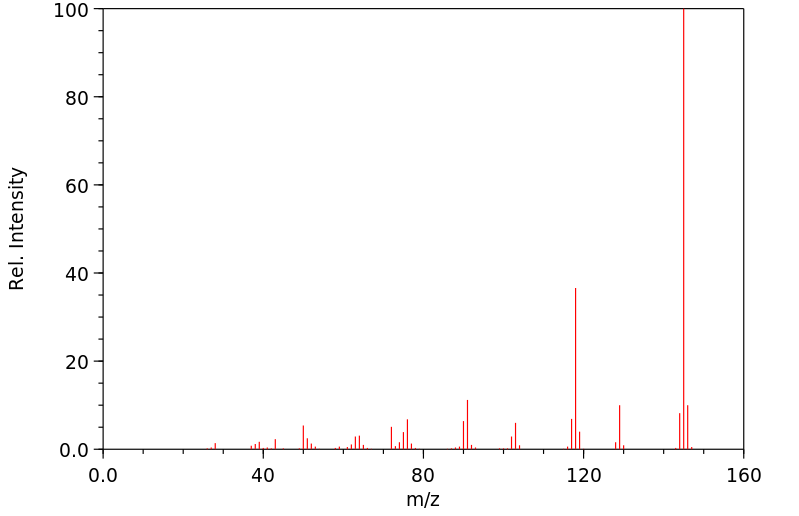
质谱MS
-
碳谱13CNMR
-
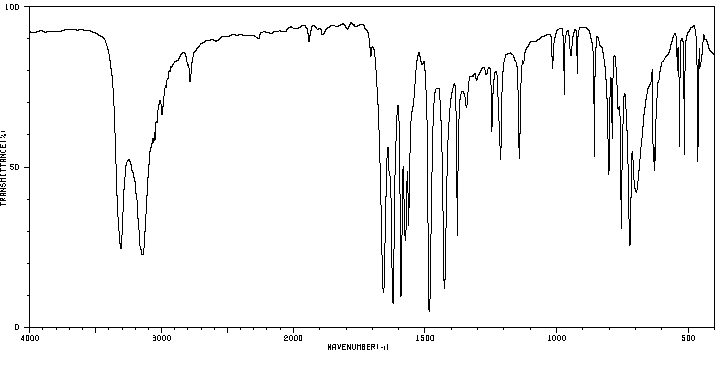
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮