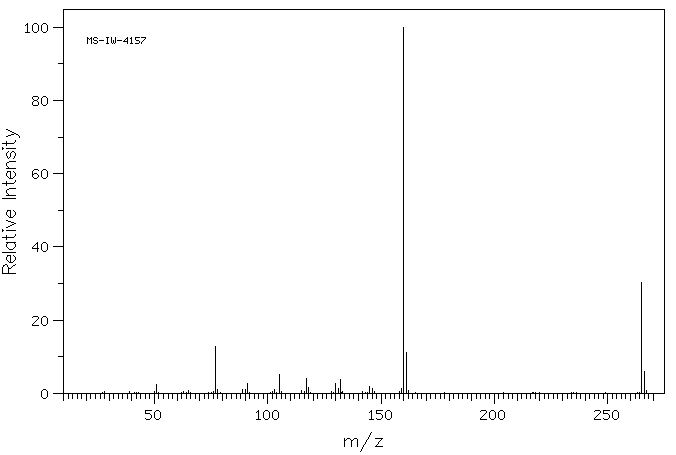
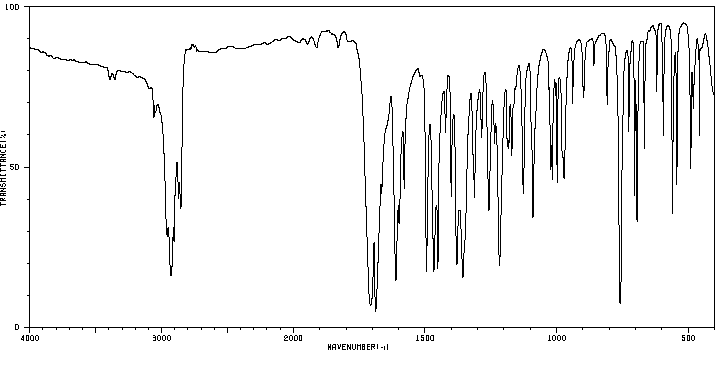
1-methyl-3-(2-oxo-2-phenylethyl)indolin-2-one | 844-49-5
中文名称
——
中文别名
——
英文名称
1-methyl-3-(2-oxo-2-phenylethyl)indolin-2-one
英文别名
1-Methyl-3-phenacyl-2-indolinone;1-methyl-3-phenacyl-3H-indol-2-one
CAS
844-49-5
化学式
C17H15NO2
mdl
——
分子量
265.312
InChiKey
UEUCHHHLQVCQMN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:20
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:37.4
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:3-甲基-1-苯基-2-丁烯 、 1-methyl-3-(2-oxo-2-phenylethyl)indolin-2-one 在 copper(l) iodide 、 2,3-二氯-5,6-二氰基-1,4-苯醌 、 4,4'-二叔丁基-2,2'-二吡啶 作用下, 反应 96.0h, 以85%的产率得到3-benzoyl-1',5-dimethyl-1-phenylspiro[cyclohex[5]ene-2,3'-indolin]-2'-one参考文献:名称:铜(I)/ DDQ介导的芳基丁烯与1,4-二酮和吲哚酮的双脱氢Diels-Alder反应。摘要:首次建立了由铜(I)/ DDQ介导的简单丁烯与1,4-二酮和吲哚酮的双脱氢Diels-Alder(DDDA)反应。此策略基于非预官能化原料的串联双脱氢/ Diels-Alder反应,其中通过在一个催化系统中活化四重惰性C(sp 3)-H键原位生成二烯和亲二烯体。DOI:10.1021/acs.orglett.0c02486
-
作为产物:描述:(Z)-1-methyl-3-(2-oxo-2-phenylethylidene)dihydroindol-2-one 在 四氯化硅 、 sodium iodide 作用下, 以 乙腈 为溶剂, 反应 0.5h, 以94%的产率得到1-methyl-3-(2-oxo-2-phenylethyl)indolin-2-one参考文献:名称:碘三氯硅烷II的还原性质的使用:α,β-不饱和酮和腈的化学选择性还原摘要:描述了一种新的合成方法,用于选择性还原α,β-不饱和酮和腈,该方法使用碘代三氯硅烷(ITCS由SiCl 4 -NaI原位生成)在适度条件下以定量收率生产相应的饱和酮和腈化合物。DOI:10.1016/0040-4039(96)00247-x
文献信息
-
N-heterocyclic carbene-catalyzed synthesis of spirocyclopentene-oxindoles from bromoenals作者:Zhao-Fei Zhang、Kun-Quan Chen、Chun-Lin Zhang、Song YeDOI:10.1039/c6cc10304a日期:——Spirocyclopentene-oxindoles were synthesized in good yields with good diastereo- and enantioselectivities via the N-heterocyclic carbene-catalyzed reaction of bromoenals and oxindoles.
-
An Expedient Synthesis of Oxindole Dimers by Direct Oxidative Dimerization of Oxindoles作者:Hyun Ju Lee、Sangku Lee、Jin Woo Lim、Jae Nyoung KimDOI:10.5012/bkcs.2013.34.8.2446日期:2013.8.20Oxindole dimers have been used as intermediates in the synthesis of various cyclotryptamine alkaloids. An efficient direct synthesis of oxindole dimers has been carried out from 3-substituted oxindoles via an oxidative dimerization using manganese(III) acetate or copper acetate/silver acetate system.
-
A Rhodium(II)‐Catalyzed Formal [4+1]‐Cycloaddition toward Spirooxindole Pyrrolone Construction Employing Vinyl Isocyanates as 1,4‐Dipoles作者:Jennifer L. Meloche、Brandon L. AshfeldDOI:10.1002/anie.201701147日期:2017.6A RhII-catalyzed, formal [4+1]-cycloaddition between diazooxindoles as electrophilic C1 synthons and 1,3-heterodienes for the construction of spirooxindole pyrrolones is described. Employing vinyl isocyanates as 1,4-dipoles, the cycloannulation occurs under relatively mild conditions and provides the corresponding pyrrolones in good to excellent yields.
-
Synthesis of 1-Alkyl-3-(2-oxo-2-aryl/alkyl-ethyl)indolin-2-ones through Gold/Brønsted Acid Relay Actions: Observation of Selective C=C Bond Cleavage of Enaminones作者:Yanzhong Li、Yulei Zhao、Yang Yuan、Lingkai Kong、Fangfang ZhangDOI:10.1055/s-0036-1588820日期:2017.8is developed. This methodology is realized by relay actions of gold and a Brønsted acid in a one-pot multistep manner. The gold(I)-catalyzed chemoselective C(sp2)-H functionalization of enaminones and Brønsted acid promoted cleavage of the C=C bond are integrated effectively. Based on the results of control experiments and ESI-MS analysis, a possible reaction mechanism is proposed. A novel gold(I)/Brønsted摘要 开发了一种新颖的金(I)/布朗斯台德酸顺序催化/促进的方法,以在温和的反应条件下合成1-烷基-3-(2-氧代-2-芳基/烷基-乙基)吲哚-2-酮。该方法是通过一锅多步的方式通过金和布朗斯台德酸的中继作用实现的。金(I)催化的化学选择性C(sp 2)-H的烯胺酮和布朗斯台德酸促进的C = C键裂解有效地整合。根据控制实验和ESI-MS分析的结果,提出了一种可能的反应机理。 开发了一种新颖的金(I)/布朗斯台德酸顺序催化/促进的方法,以在温和的反应条件下合成1-烷基-3-(2-氧代-2-芳基/烷基-乙基)吲哚-2-酮。该方法是通过一锅多步的方式通过金和布朗斯台德酸的中继作用实现的。金(I)催化的化学选择性C(sp 2)-H的烯胺酮和布朗斯台德酸促进的C = C键裂解有效地整合。根据控制实验和ESI-MS分析的结果,提出了一种可能的反应机理。
-
Tosylhydrazine-promoted self-conjugate reduction–Michael/aldol reaction of 3-phenacylideneoxindoles towards dispirocyclopentanebisoxindole derivatives作者:Sayan Pramanik、Chhanda MukhopadhyayDOI:10.3762/bjoc.18.49日期:——
An efficient tosylhydrazine-mediated conjugate reduction of 3-phenacylideneoxindole and sequential Michael/intramolecular aldol reaction is reported under base-catalyzed conditions towards the formation of densely substituted dispirocyclopentanebisoxindole derivatives. The reaction proceeded in a diastereoselective manner to afford four chiral stereocenters. The method also has advantages of wide substrate scope, readily available starting materials and operational simplicity through one pot reaction.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷