甘菊环-2-胺; 环戊并环庚五烯-2-胺; 薁-2-胺 | 50472-20-3
中文名称
甘菊环-2-胺; 环戊并环庚五烯-2-胺; 薁-2-胺
中文别名
甘菊环-2-胺; 环戊并环庚五烯-2-胺; 薁-2-胺;环戊并环庚五烯-2-胺;甘菊环-2-胺;环戊并环庚五烯-2-胺;薁-2-胺;甘菊环-2-胺
英文名称
2-aminoazulene
英文别名
azulen-2-ylamine;Azulen-2-ylamin;Azulen-2-amine
CAS
50472-20-3
化学式
C10H9N
mdl
MFCD18072913
分子量
143.188
InChiKey
URSBMMUQJIMLKA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93-94℃
-
沸点:307℃
-
密度:1.137
-
闪点:157℃
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2921499090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:2-8°C
SDS
反应信息
-
作为反应物:描述:甘菊环-2-胺; 环戊并环庚五烯-2-胺; 薁-2-胺 在 palladium on activated charcoal 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 xylene 、 苯 为溶剂, 反应 7.25h, 生成 12-Azatetracyclo[11.6.1.02,11.03,9]icosa-1(20),2,4,6,8,10,12-heptaene参考文献:名称:On the Reactions of (Vinylimino)phosphoranes and Related Compounds. 27. A Short New Synthesis of Azuleno[2,1-b]pyridines and Azuleno-Annulated [n](2,4)Pyridinophanes摘要:A short new synthesis of azuleno[2,1-b]pyridines 6a-g consists of the reaction of 2-aminoazulene (4) with acyclic alpha,beta-unsaturated ketones and aldehydes in an enamine-alkylation process, subsequent condensation of the amino group with a carbonyl function, and dehydrogenation in the presence of Pd/C. Similarly, the reaction of 4 with alpha,beta-unsaturated cycloalkenones gives azuleno-annulated [n](2,4)pyridinophanes 12a-c (n = 9-7) and dihydrogenated analogue 11d (n = 6). Compound 11d is converted to azuleno-annulated [6](2,5)pyridinophane 12d by treatment with DDQ. H-1 NMR spectroscopy at various temperatures shows dynamic behavior for the oligomethylene chains of [7]- and [6](2,4)pyridinophanes 12c,d. The energy barriers (DELTAG(c)double dagger) for the bridge flipping are 10.8 kcal/mol (T(c), -30-degrees-C) for 12c and 18.1 kcal/mol (T(c), 90-degrees-C) for 12d. Although the strain of 12c,d increases as the chainlength becomes shorter, the pyridine ring of 12c,d can flex more easily than that of the corresponding unannulated [n] (2,4)pyridinophanes. The deformation of the pyridine ring of 12a-d is also suggested by red shifts of the UV and H-1 NMR spectra. The pK(a) values of 12a-d are independent of the size of the methylene bridge, suggesting that the energy differences between the protonated and nonprotonated forms are almost the same for 12a-d.DOI:10.1021/jo00085a018
-
作为产物:描述:参考文献:名称:The Synthesis of Azulene Derivatives from Troponoids摘要:DOI:10.1246/bcsj.35.1179
文献信息
-
Coupling Reaction of Azulenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolanes with Haloazulenes作者:Kei Kurotobi、Hiroshi Tabata、Masato Miyauchi、Toshihiro Murafuji、Yoshikazu SugiharaDOI:10.1055/s-2002-31947日期:——In order to study the physicochemical properties of azulene oligomers, the synthesis and a coupling reaction of 2-(2-amino-1,3-bisethoxycarbonyl-6-azulenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1) and 2-(2-azulenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (2) were examined.
-
An Easy Access to 2-Substituted Azulenes from Azulene-2-boronic Acid Pinacol Ester作者:Toshihiro Murafuji、Yoshikazu Sugihara、Masayuki Fujinaga、Kouichi Suetake、Kazuhiro Gyoji、Kei KurotobiDOI:10.1055/s-0028-1083224日期:2008.12Azulene-2-boronic acid pinacol ester was conveniently transformed into 2-substituted azulenes bearing carboxyl, formyl, ester, or amino groups, which are difficult to access by conventional methods. Furthermore, the azulene-2-carboxylic acid thus synthesized was subjected to the Ugi four-component condensation to obtain a dipeptide-type product containing an azulene skeleton.
-
Reactions of 2-Aminoazulenes with Electron-Deficient Acetylenes作者:Noritaka AbeDOI:10.1246/bcsj.64.2393日期:1991.82-Aminoazulene (1a) reacted with dimethyl acetylenedicarboxylate (DMAD) to give dimethyl 1,2-azulenedicarboxylate, tetramethyl 2,2′-(2-amino-1,3-azulenediyl)bis[fumarate], and dimethyl 2-(4-methoxycarbonyl-2-oxo-l,2-dihydroazuleno[2,1-b]pyridin-10-yl)fumarate. The reaction of ethyl 2-amino-1-azulenecarboxylate (1b) with DMAD gave dimethyl 2-(2-amino-3-ethoxycarbonyl-1-azulenyl)fumarate and 10-ethyl 4-methyl 2-oxo-1,2-dihydroazuleno[2,1-b]pyridine-4,10-dicarboxylate (8a). The treatment of 8a with phosphoryl chloride gave the 2-chloroazuleno[2,1-b]pyridine derivative. Reactions of 1a and 1b with methyl propiolate gave corresponding Michael adducts and the azuleno[2,1-b]pyridin-3(4H)-one derivatives. Reactions of 1a and 1b with dibenzoylacetylene gave 4-benzoyl-2-phenylazuleno[2,1-b]pyridine derivatives.2-氨基甘菊环 (1a) 与双甲基乙炔二羧酸酯 (DMAD) 反应,生成双甲基1,2-甘菊环二羧酸酯、四甲基2,2′-(2-氨基-1,3-甘菊环二基)双[富马酸酯]以及双甲基2-(4-甲氧羰基-2-氧-1,2-二氢甘菊环[2,1-b]吡啶-10-基)富马酸酯。乙基2-氨基-1-甘菊环羧酸酯 (1b) 与DMAD反应,生成双甲基2-(2-氨基-3-乙氧羰基-1-甘菊环基)富马酸酯和10-乙基4-甲基2-氧-1,2-二氢甘菊环[2,1-b]吡啶-4,10-二羧酸酯 (8a)。用磷酰氯处理8a得到2-氯甘菊环[2,1-b]吡啶衍生物。1a和1b与甲基丙炔酸酯的反应生成相应的Michael加合物和甘菊环[2,1-b]吡啶-3(4H)-酮衍生物。1a和1b与二苯甲酰乙炔的反应生成4-苯甲酰基-2-苯基甘菊环[2,1-b]吡啶衍生物。
-
Preparation, characterization, and cycloaddition reaction of the heterocumulenes attached directly to azulenes. An efficient strategy for the preparation of azulene-substituted heterocycles作者:Shunji Ito、Tetsuo Okujima、Chizuko Kabuto、Noboru MoritaDOI:10.1016/s0040-4020(03)00637-9日期:2003.6Preparation and cycloaddition reaction of novel azulene-substituted N-sulfinylamines 1 and 2 are reported. The influence of the –NSO group on the UV–vis and NMR spectra of the azulene ring to which it is bonded is discussed. X-ray crystal analysis of 1 revealed the syn-configuration and the twisted structure of the N-sulfinylamine moiety. The synthetic utility of 1 and 2 have been explored by the报道了新颖的氮杂取代的N-亚磺酰基胺1和2的制备和环加成反应。讨论了–NSO基团对与其键合的z环的UV-vis和NMR光谱的影响。的X射线晶体分析1揭示了顺式构型和所述的扭曲结构Ñ -sulfinylamine部分。已通过环加成反应探索了1和2的合成效用,以在高压条件下提供新型的氮杂取代的杂环。我们还在本文中描述了相关的2-氮杂烯基异硫氰酸酯的合成和一些性质。
-
Synthesis and photophysical properties of azuleno[1′,2′:4,5]pyrrolo[2,1-b]quinazoline-6,14-diones: Azulene analogs of tryptanthrin作者:Chieko Kogawa、Asuka Fujiwara、Ryuta Sekiguchi、Taku Shoji、Jun Kawakami、Masaaki Okazaki、Shunji ItoDOI:10.1016/j.tet.2018.10.020日期:2018.12Azulene analog of tryptanthrin, azuleno[1′,2′:4,5]pyrrolo[2,1-b]quinazoline-6,14-dione, was successfully prepared by the condensation reaction of azuleno[2,1-b]pyrrole-2,3-dione with isatoic anhydride in the presence of sodium hydride or diisopropylethylamine (DIPEA). Its 2-halo derivatives were also obtained in high yields by the condensation reaction with 5-haloisatoic anhydrides in the presence通过azuleno [2,1- b ]吡咯的缩合反应成功制备了色胺酮的azulno类似物azuleno [1',2':4,5]吡咯并[2,1 - b ]喹唑啉-6,14-二酮。在氢化钠或二异丙基乙胺(DIPEA)的存在下,将-2,3-二酮与isatoic酸酐一起使用。在DIPEA存在下,通过与5-卤代氨基甲酸酯的缩合反应也可以高产率获得其2-卤代衍生物。通过使用N-溴琥珀酰亚胺(NBS)或N进行卤化可以揭示出对亲电试剂的反应性-碘代琥珀酰亚胺(NIS)以高产率提供12-卤代衍生物。在卤代衍生物中,2-碘和12-碘衍生物在Pd催化的Sonogashira交叉偶联条件下具有足够的反应活性,可提供苯基乙炔基衍生物。在苯基乙炔基衍生物内,通过与TCNE反应,仅12-苯基乙炔基衍生物被转化为其1,1,4,4-四氰基丁二烯(TCBD)衍生物。通过光谱分析和伏安分析对新型的色胺酮类天青石类似物的两性氧化还原特性进行了表征。
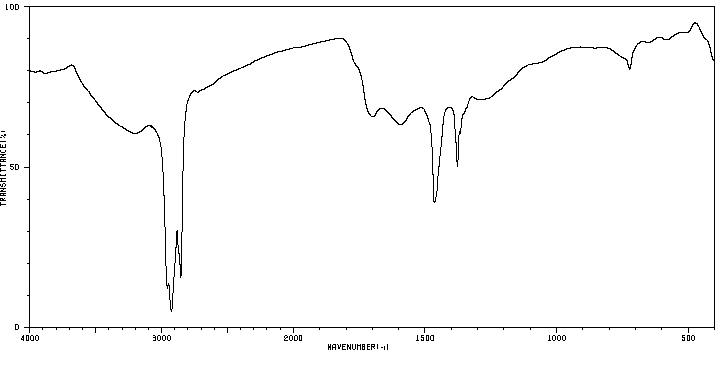
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-