2-溴丙酸-N-丁酯 | 41145-84-0
中文名称
2-溴丙酸-N-丁酯
中文别名
2-溴丙酸丁酯;2-溴丙酸正丁基酯
英文名称
2-bromopropionic acid n-butyl ester
英文别名
butyl 2-bromopropanoate;2-bromopropionic acid butyl ester;butyl bromopropionate;2-Brom-propionsaeure-butylester;dl-α-Brom-propionsaeure-butylester;n-butyl α-bromopropionate;butyl α-bromopropionate;DL-butyl-(2-bromo propionate);DL-Butyl-(2-brom-propionat);2-bromo-propanoic acid, butyl ester;butyl 2-bromopropionate
CAS
41145-84-0
化学式
C7H13BrO2
mdl
——
分子量
209.083
InChiKey
INMAEXGIVZJYIJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:196 °C
-
密度:1.28
-
保留指数:1102;1098
-
稳定性/保质期:
常规情况下不会分解,没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915900090
-
储存条件:密封、阴凉、干燥保存。
SDS
2-溴丙酸丁酯 修改号码:5
模块 1. 化学品
产品名称: Butyl 2-Bromopropionate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
2-溴丙酸丁酯 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-溴丙酸丁酯
百分比: >95.0%(GC)
CAS编码: 41145-84-0
俗名: 2-Bromopropionic Acid Butyl Ester
分子式: C7H13BrO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
2-溴丙酸丁酯 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 196 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.28
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
2-溴丙酸丁酯 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Butyl 2-Bromopropionate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
2-溴丙酸丁酯 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-溴丙酸丁酯
百分比: >95.0%(GC)
CAS编码: 41145-84-0
俗名: 2-Bromopropionic Acid Butyl Ester
分子式: C7H13BrO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
2-溴丙酸丁酯 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 196 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.28
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
2-溴丙酸丁酯 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:2-溴丙酸-N-丁酯 在 sodium 、 对甲苯磺酸 作用下, 以 正丁醇 为溶剂, 生成 α-Methyl-β-<β-carbomethoxy-aethyl>-laevulinsaeure-butylester参考文献:名称:L'vova,S.D. et al., Journal of Organic Chemistry USSR (English Translation), 1965, vol. 1, # 9, p. 1578 - 1581摘要:DOI:
-
作为产物:描述:参考文献:名称:用α-溴羰基化合物进行镍催化的杂环直接烷基化:2-吡啶酮的C3选择性官能化摘要:镍:Ni(cod)2 / dppp催化剂体系促进富电子杂环与α-溴羰基化合物的直接烷基化,并涉及烷基自由基中间体(请参见方案; cod = 1,5-环辛二烯,dppp = 1 ,3-双(二苯基膦基)丙烷)。这种均质的自由基芳香取代(HAS)型反应可实现2-吡啶酮的C3选择性直接官能化。DOI:10.1002/chem.201301350
文献信息
-
Effect of Optically Active Ethyl 2-Phthalimidooxypropionate on the Growth of Cress,<i>Lepidium sativum</i>作者:Yukinori TAKEKIDA、Momotoshi OKAZAKI、Yoshihiro SHUTODOI:10.1271/bbb.63.1831日期:1999.1The effects of 2-phthalimidooxyalkanoic acid derivatives on the germination and root-growth of cress were examined. Since 2-phthalimidooxypropionates were most effective, the optically active ethyl esters were prepared. As the result of biological testing, the (S)-(-)-isomer exhibited stronger activity than the (R)-(+)-isomer. This result is contrary to those from commercial herbicides with similar structures, phenoxy- and oxyphenoxy-propionate-type compounds, where the (R)-isomers are generally known to be the active principles.
-
[EN] PROTECTED ORGANOBORONIC ACIDS WITH TUNABLE REACTIVITY, AND METHODS OF USE THEREOF<br/>[FR] ACIDES ORGANOBORONIQUES PROTÉGÉS À RÉACTIVITÉ AJUSTABLE ET LEURS PROCÉDÉS D'UTILISATION申请人:UNIV ILLINOIS公开号:WO2017027435A1公开(公告)日:2017-02-16Disclosed are a range of protected organoboronic acid reagents useful in the modular assembly of complex organic compounds. The reactivities of the protected organoboronic acid reagents may be varied predictably by changes to the number and identities of their substituents. Also disclosed are methods of using the protected organoboronic acid reagents in the synthesis of organic compounds.
-
一种芴基团和羧酸酯结合的化合物及其应用
-
Cooperative Ligand-Promoted P<sup>(III)</sup>-Directed Ruthenium-Catalyzed Remote <i>Meta</i>-C–H Alkylation of Tertiary Phosphines作者:Zheng-Xin Zhou、Jia-Wei Li、Liang-Neng Wang、Ming Li、Yue-Jin Liu、Ming-Hua ZengDOI:10.1021/acs.orglett.1c00237日期:2021.3.19ruthenium-catalyzed meta-selective C–H activation of phosphines by using intrinsic P(III) as a directing group. 2,2,6,6-Tetramethylheptane-3,5-dione acts as the ligand and exhibits an excellent performance in boosting the meta-alkylation. The protocol allows an efficient and straightforward synthesis of meta-alkylated tertiary phosphines. Several meta-alkylated phosphines were evaluated for Pd-catalyzed Suzuki coupling在本文中,我们公开了钌催化的元通过使用固有的P.膦-选择性C-H活化(III)作为定向基团。2,2,6,6-四甲基庚烷-3,5-二酮充当配体并在促进间位烷基化方面表现出出色的性能。该方案允许间位烷基化的叔膦的有效而直接的合成。评估了几种间位烷基化的膦在Pd催化的Suzuki偶联中的作用,发现其优于市售的邻位取代的膦。通过双官能化膦的合成进一步证明了该方法的实用性。
-
Synthesis of [13C3]-B6 Vitamers Labelled at Three Consecutive Positions Starting from [13C3]-Propionic Acid作者:Thomas Bachmann、Michael RychlikDOI:10.3390/molecules23092117日期:——[13C₃]-labelled vitamers (PN, PL and PM) of the B6 group were prepared starting from [13C₃]-propionic acid. [13C₃]-PN was synthesized in ten linear steps with an overall yield of 17%. Hereby, higher alkyl homologues of involved esters showed a positive impact on the reaction outcome of the intermediates in the chosen synthetic route. Oxidation of [13C₃]-PN to [13C₃]-PL was undertaken using potassium
表征谱图
-
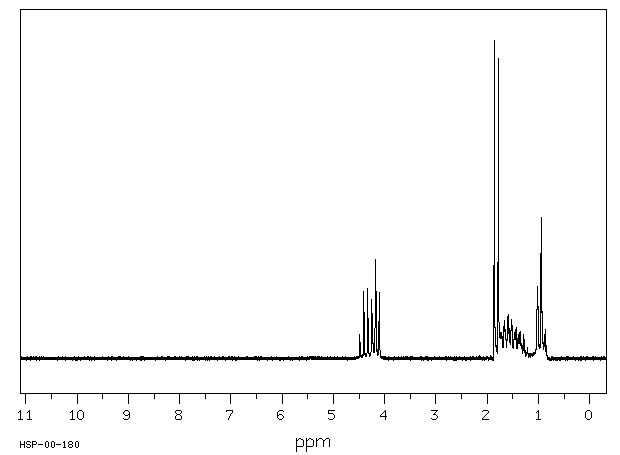
氢谱1HNMR
-
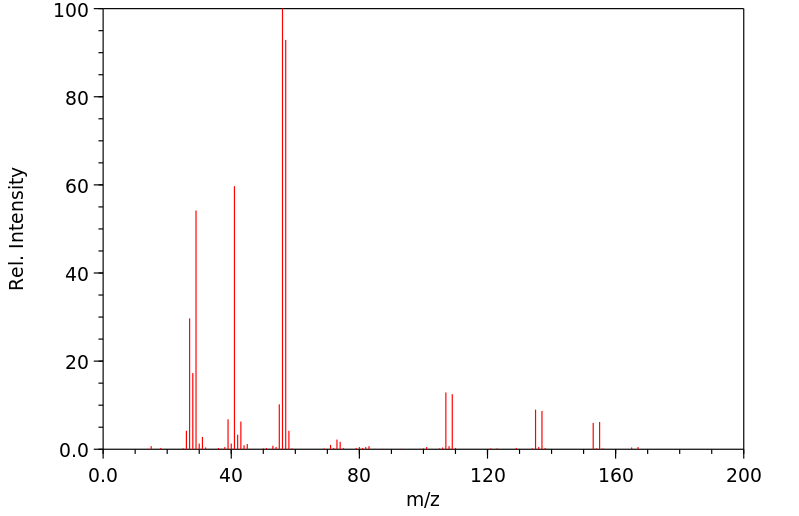
质谱MS
-
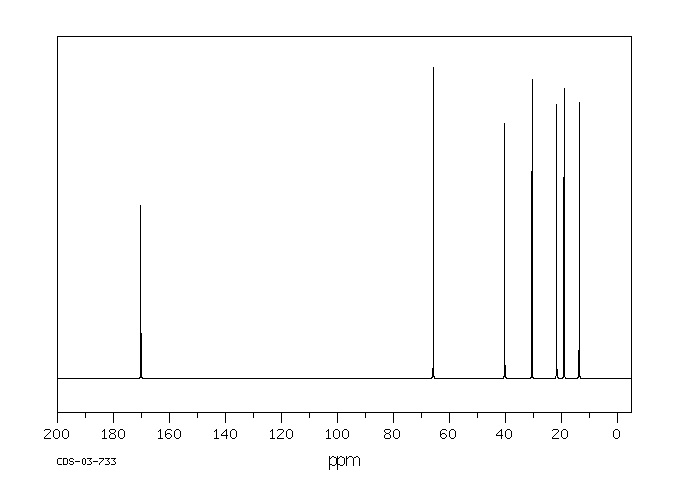
碳谱13CNMR
-
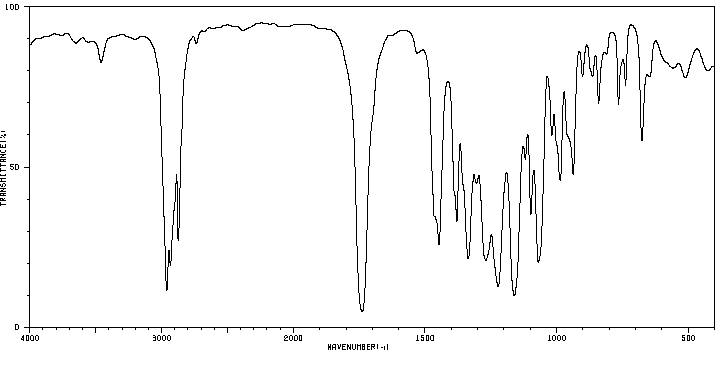
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸