2-甲基-1-辛烯-3-炔 | 17603-76-8
中文名称
2-甲基-1-辛烯-3-炔
中文别名
——
英文名称
2-methyl-1-octen-3-yne
英文别名
2-Methyl-octen-(1)-in-(3);2-methyloct-1-en-3-yne
CAS
17603-76-8
化学式
C9H14
mdl
MFCD00041648
分子量
122.21
InChiKey
FPKTZBDAOSTAHN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:67-69°C 36mm
-
密度:0,775 g/cm3
-
保留指数:981
-
稳定性/保质期:
存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Silver(I)-Catalyzed Cascade: Direct Access to Furans from Alkynyloxiranes摘要:Functionalized furans are conveniently formed by a new silver(I)-catalyzed reaction of alk-1-ynyl oxiranes in the presence of p-toluenesulfonic acid and methanol. Evidence supported a cascade mechanism.DOI:10.1021/jo900483m
-
作为产物:描述:参考文献:名称:Ioffe,B.V. et al., Journal of Organic Chemistry USSR (English Translation), 1968, vol. 4, p. 1689 - 1694摘要:DOI:
文献信息
-
Regio- and Stereoselective Synthesis of Enol Carboxylate, Phosphate, and Sulfonate Esters via Iodo(III)functionalization of Alkynes作者:Chang-Sheng Wang、Ploypailin Siew Ling Tan、Wei Ding、Shingo Ito、Naohiko YoshikaiDOI:10.1021/acs.orglett.1c04123日期:2022.1.14synthesized through regio- and stereoselective iodo(III)functionalization of alkynes. The combination of chlorobenziodoxole and silver salt has proven to generate a versatile cationic iodine(III) electrophile to activate alkynes and engage various carboxylic acids, triethyl phosphate, and p-toluenesulfonic acid as nucleophiles. The β-iodo(III)enol esters serve as starting materials for the synthesis of multisubstituted
-
Nickel‐Catalyzed Carbofluoroalkylation of 1,3‐Enynes to Access Structurally Diverse Fluoroalkylated Allenes作者:Kai‐Fan Zhang、Kang‐Jie Bian、Chao Li、Jie Sheng、Yan Li、Xi‐Sheng WangDOI:10.1002/anie.201813184日期:2019.4A nickel‐catalyzed 1,4‐carbofluoroalkylation of 1,3‐enynes to access structurally diverse fluoroalkylated allenes has been established. This method has demonstrated high catalytic reactivity, mild reaction conditions, broad substrate scope, and excellent functional‐group tolerance. The key to success is the use of a nickel catalyst to generate different fluoroalkyl radicals from readily available and
-
Beyond the Limits: Palladium-N-Heterocyclic Carbene-Based Catalytic System Enables Highly Efficient [4+2] Benzannulation Reactions作者:Olga V. Zatolochnaya、Alexey V. Galenko、Vladimir GevorgyanDOI:10.1002/adsc.201100983日期:2012.4.16A highly efficient catalytic system for the palladium‐catalyzed [4+2] benzannulation reaction of enynes and enynophiles has been developed. The use of an N‐heterocyclic carbene‐based palladium precursor allowed us to achieve turnover numbers up to 1800. The new catalytic system has enabled an expansion of the scope of the [4+2] homo‐benzannulation reaction.
-
Remarkably Selective Formation of Allenyl and Dienyl Alcohols via Ni-Catalyzed Coupling Reaction of Conjugated Enyne, Aldehyde, and Organozinc Reagents作者:Masanari Kimura、Yasuyuki Mori、Toshiki Kawabata、Gen OnoderaDOI:10.1055/s-0035-1560448日期:——conjugated enynes, aldehydes, and organozinc reagents to form unsaturated alcohols. Ligand effects dramatically control the regioselectivity in these Ni-catalyzed MCRs, leading to the selective formation of allenyl alcohols and conjugated dienyl alcohols. A nickel catalyst promotes the multi-component reactions (MCRs) of conjugated enynes, aldehydes, and organozinc reagents to form unsaturated alcohols
-
Ni-Catalyzed Three-component Coupling Reaction of Conjugated Enyne, Carbonyls, and Dimethylzinc to Construct Allenyl Alcohols作者:Yasuyuki Mori、Gen Onodera、Masanari KimuraDOI:10.1246/cl.130865日期:2014.1.5Nickel catalyzes the three-component coupling reaction of dimethylzinc, enyne, and carbonyls to provide tetrasubstituted allenyl alcohols. Diethylzinc and diphenylzinc can participate in similar coupling reactions to provide the corresponding allenyl alcohols in reasonable yields.
表征谱图
-
氢谱1HNMR
-
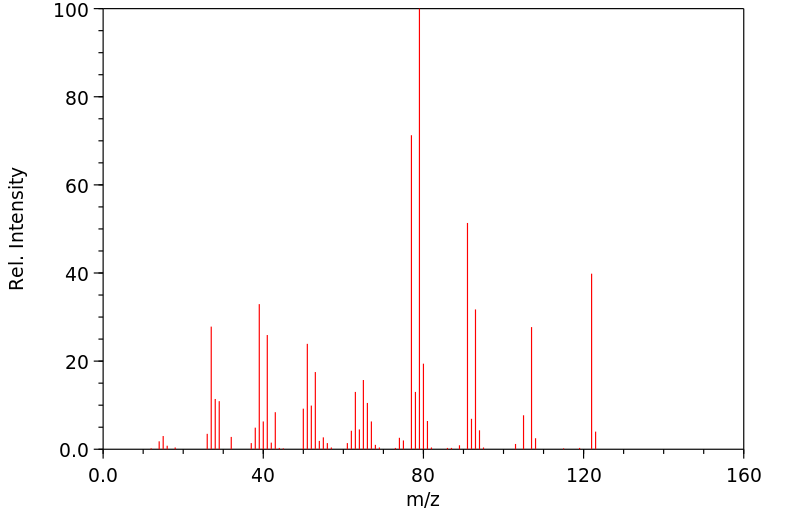
质谱MS
-
碳谱13CNMR
-
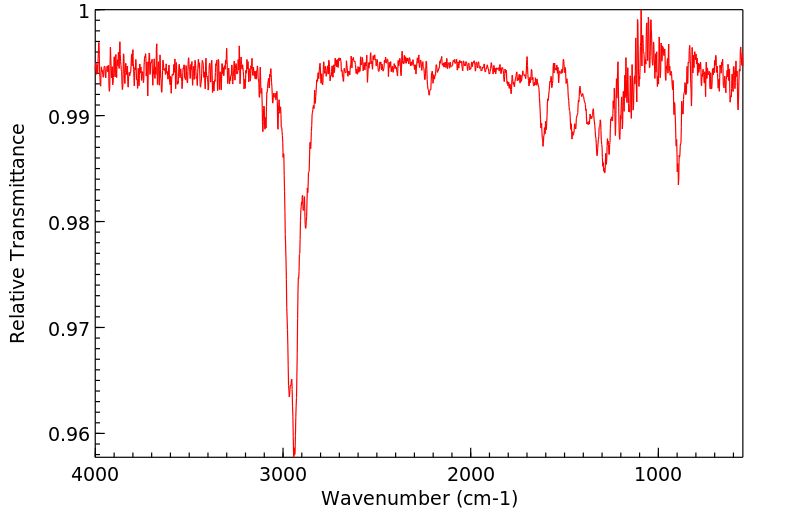
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-