2-甲基-5-壬酮 | 22287-02-1
中文名称
2-甲基-5-壬酮
中文别名
——
英文名称
2-methyl-5-nonanone
英文别名
2-methyl-nonan-5-one;Butyl-isopentyl-keton;2-Methyl-nonan-5-on;i-Amyl-n-butyl-keton;2-Methyl-5-nonanon;2-methylnonan-5-one
CAS
22287-02-1
化学式
C10H20O
mdl
——
分子量
156.268
InChiKey
YITHWSJQOVKDGI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-3.45°C (estimate)
-
沸点:203.55°C
-
密度:0.8213
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:11
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.9
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2914190090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-己酮 2-Hexanone 591-78-6 C6H12O 100.161
反应信息
-
作为反应物:描述:2-甲基-5-壬酮 在 溴 、 silver carbonate 作用下, 以 正戊烷 为溶剂, 反应 0.67h, 生成 2,2,5-trimethyl-5-butyltetrahydrofuran参考文献:名称:通过溴-银-盐反应由失活的脂肪族醇形成四氢呋喃的选择性摘要:溴-碳酸银与脂肪族醇的反应的研究通常是非常具体的,在脂肪族醇中可能发生分子内δ-H竞争。叔-仲醇和叔-仲醇均优先损失δ-氢,从而生成取代度最高的环醚。例如,2,5-二甲基-2-辛醇仅产生2,2,5-三甲基-5-丙基四氢呋喃作为环醚产物;未检测到2-甲基-2-异戊基四氢呋喃。DOI:10.1016/s0040-4020(01)96881-4
-
作为产物:描述:参考文献:名称:Powell; Hagemann, Journal of the American Chemical Society, 1944, vol. 66, p. 374摘要:DOI:
文献信息
-
Direct Synthesis of 1,4-Diols from Alkenes by Iron-Catalyzed Aerobic Hydration and CH Hydroxylation作者:Takuma Hashimoto、Daisuke Hirose、Tsuyoshi TaniguchiDOI:10.1002/anie.201308675日期:2014.3.3Various 1,4‐diols are easily accessible from alkenes through iron‐catalyzed aerobic hydration. The reaction system consists of a user‐friendly iron phthalocyanine complex, sodium borohydride, and molecular oxygen. Furthermore, the effect of additional ligands on the iron complex was examined for a model reaction. The second hydroxy group is installed by direct C(sp3)H oxygenation, which is based on
-
Iron‐Catalysed Remote C(sp <sup>3</sup> )−H Azidation of <i>O</i> ‐Acyl Oximes and <i>N</i> ‐Acyloxy Imidates Enabled by 1,5‐Hydrogen Atom Transfer of Iminyl and Imidate Radicals: Synthesis of γ‐Azido Ketones and β‐Azido Alcohols作者:Rubén O. Torres‐Ochoa、Alexandre Leclair、Qian Wang、Jieping ZhuDOI:10.1002/chem.201901079日期:2019.7.17acetylacetonate [Fe(acac)3], the reaction of structurally diverse ketoxime esters with trimethylsilyl azide (TMSN3) afforded γ‐azido ketones in good to excellent yields. This unprecedented distal γ‐C(sp3)−H bond azidation reaction went through a sequence of reductive generation of an iminyl radical, 1,5‐hydrogen atom transfer (1,5‐HAT) and iron‐mediated redox azido transfer to the translocated carbon radical
-
Compositions and Methods for Fermentation of Biomass申请人:Parekh Sarad公开号:US20100268000A1公开(公告)日:2010-10-21In one aspect, this invention relates to production of useful fermentation end-products from biomass through simultaneous hydrolysis and fermentation by a microorganism, such as Clostridium phytofermentans . The invention also relates to the development of a process for efficient pretreatment and conversion of lignocellulosic biomass to end-products with high conversion efficiency (yield). In another aspect, methods for producing a fermentation end-product by fermenting hexose (C6) and pentose (C5) sugars with a microorganism, such as Clostridium phytofermentans are disclosed herein.
-
BIOFUEL PRODUCTION申请人:Yoshikuni Yasuo公开号:US20090139134A1公开(公告)日:2009-06-04Methods, enzymes, recombinant microorganism, and microbial systems are provided for converting polysaccharides, such as those derived from biomass, into suitable monosaccharides or oligosaccharides, as well as for converting suitable monosaccharides or oligosaccharides into commodity chemicals, such as biofuels. Commodity chemicals produced by the methods described herein are also provided. Commodity chemical enriched, refinery-produced petroleum products are also provided, as well as methods for producing the same.
-
METHODS AND COMPOSITIONS FOR PRODUCING CHEMICAL PRODUCTS FROM C. PHYTOFERMENTANS申请人:Schmalisch Matthias公开号:US20110183382A1公开(公告)日:2011-07-28This invention provides systems and methods for the production of compounds by C. phytofermentans. C. phytofermentans is genetically-engineered for hydrolysis and fermentation of carbonaceous biomass to synthesize compounds of commercial value.
表征谱图
-
氢谱1HNMR
-
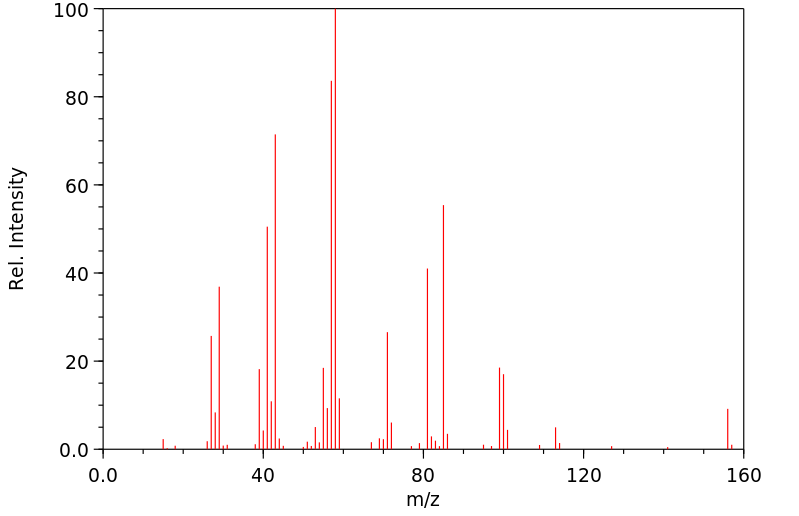
质谱MS
-
碳谱13CNMR
-
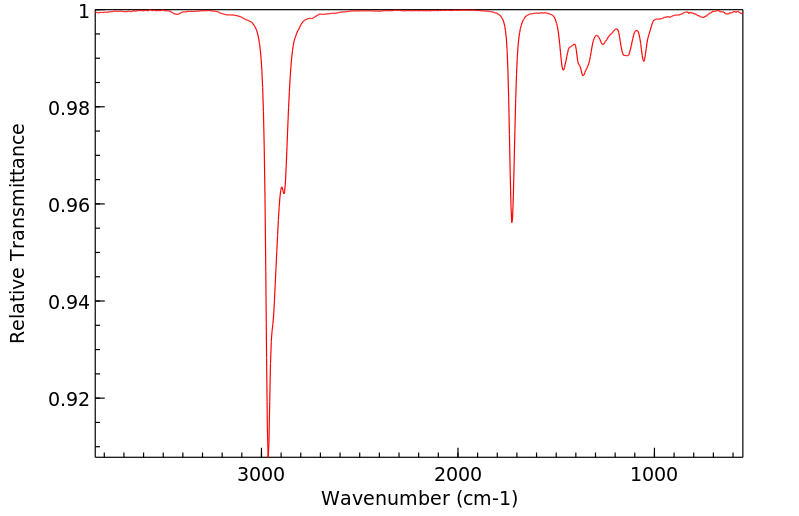
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷