2-甲基-7-十八炔 | 35354-38-2
中文名称
2-甲基-7-十八炔
中文别名
——
英文名称
2‐methyl‐7‐octadecyne
英文别名
2-methyl-7-octadecyne;2-methyloctadec-7-yne;2-methyl-octadec-7-yne;2-methyl-octadecyne;2-Methyl-octadecin-(7);2-Methyl-7-octadecin;7-Octadecyne, 2-methyl-
CAS
35354-38-2
化学式
C19H36
mdl
——
分子量
264.495
InChiKey
WQMKDHREUPHQQE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:131-133 °C(Press: 1 Torr)
-
密度:0.811±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):9
-
重原子数:19
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:0.894
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 7-甲基-1-辛炔 7-methyl-1-octyne 118864-33-8 C9H16 124.226
反应信息
-
作为反应物:描述:2-甲基-7-十八炔 在 9-borabicyclo[3.3.1]nonane dimer 、 p-(methoxycarbonyl)perbenzoic acid 、 溶剂黄146 作用下, 以 氯仿 为溶剂, 反应 30.0h, 生成 (-)-disparlure参考文献:名称:昆虫信息素及其类似物。八。2-methyloctadec-7-ene 和 2-methyl-7,8-epoxyoctadecane 的 (Z) 和 (E) 异构体的合成摘要:已开发出一种用于合成外消旋 (Z)-disparlure 的高度立体有择的方法,该方法基于在 9-硼双环 [3.3.1] 壬烷的帮助下还原 2-methyloctadec-7-yne 和所得的环氧化(Z)-2-methyloctadec-7-ene 与对甲氧基羰基过苯甲酸。给出了 2-methyloctadec-7-ene 和 2-methyl-7,8-epoxyoctadecane 的 (Z) 和 (E) 异构体的 13 C NMR 光谱,它们明确地证实了这些化合物的结构。已经确定 (E)-2-methyloctadec-7-ene 表现出中等的引诱活性,而 (Z) 异构体不吸引吉普赛蛾。添加 5-25% 的 (E)-disparlure 会增加 (Z)-disparlure 的生物活性。DOI:10.1007/bf00576092
-
作为产物:描述:参考文献:名称:Jonas, Jaroslav; Slanina, Pavel; Humpa, Otakar, Collection of Czechoslovak Chemical Communications, 1988, vol. 53, # 4, p. 857 - 859摘要:DOI:
文献信息
-
Zakharkin, L. I.; Zhigareva, G. G., Journal of Organic Chemistry USSR (English Translation), 1981, vol. 17, p. 1831 - 1833作者:Zakharkin, L. I.、Zhigareva, G. G.DOI:——日期:——
-
Tritium-labeled enantiomers of disparlure. Synthesis and in vitro metabolism作者:Glenn D. Prestwich、Steven M. Graham、Jing Wen Kuo、Richard G. VogtDOI:10.1021/ja00184a035日期:1989.1
-
JONAS, JAROSLAV;SLANINA, PAVEL;HUMPA, OTAKAR作者:JONAS, JAROSLAV、SLANINA, PAVEL、HUMPA, OTAKARDOI:——日期:——
-
ZAXARKIN, L. I.;ZHIGAREVA, G. G., ZH. ORGAN. XIMII, 1981, 17, N 10, 2051-2054作者:ZAXARKIN, L. I.、ZHIGAREVA, G. G.DOI:——日期:——
-
ODINOKOV, V. N.;BALEZINA, G. G.;DZHEMILEV, U. M.;ISHMURATOV, G. YU.;AMIRX+, XIMIYA PRIROD. SOEDIN., 1983, N 5, 630-634作者:ODINOKOV, V. N.、BALEZINA, G. G.、DZHEMILEV, U. M.、ISHMURATOV, G. YU.、AMIRX+DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
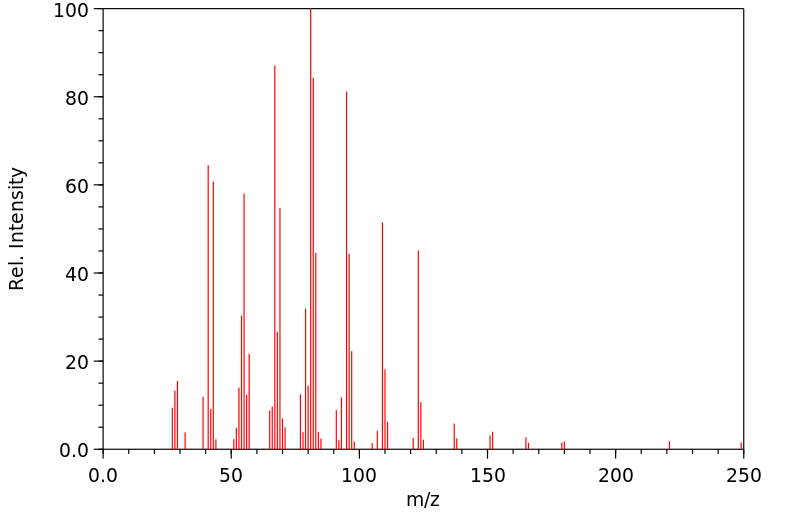
质谱MS
-
碳谱13CNMR
-
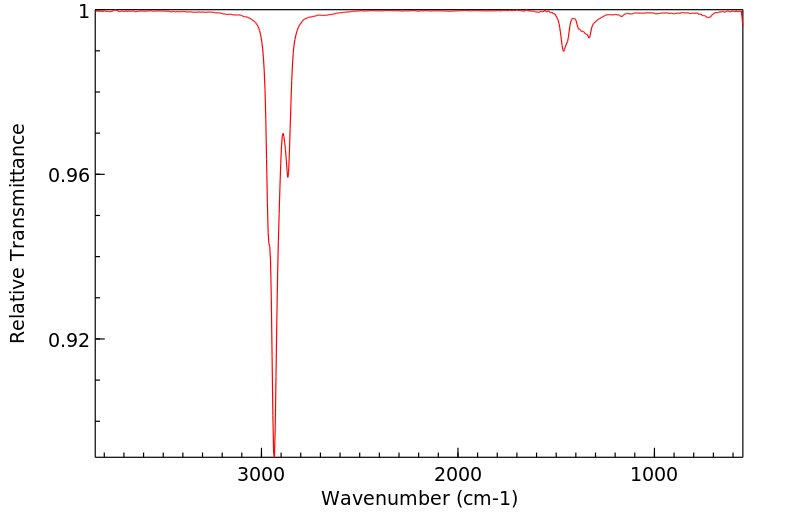
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-