2-甲基苯氧基乙酸 | 1878-49-5
中文名称
2-甲基苯氧基乙酸
中文别名
(2-甲苯氧基)乙酸;2-甲苯氧基乙酸
英文名称
o-methylphenoxyacetic acid
英文别名
2-Methylphenoxyacetic Acid;2-(2-Methylphenoxy)acetic acid
CAS
1878-49-5
化学式
C9H10O3
mdl
MFCD00014354
分子量
166.177
InChiKey
QJVXBRUGKLCUMY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:155-157 °C(lit.)
-
沸点:>150 °C
-
密度:1.179±0.06 g/cm3(Predicted)
-
溶解度:可溶于乙腈(少许)、DMSO(少许)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2918990090
-
RTECS号:AJ7573000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
| Name: | (2-Methylphenoxy)acetic acid, 99% Material Safety Data Sheet |
| Synonym: | None. |
| CAS: | 1878-49-5 |
Synonym: None.
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1878-49-5 | (2-Methylphenoxy)acetic acid | 99 | 217-517-4 |
Risk Phrases: 36/37/38
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Irritating to eyes, respiratory system and skin. Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
No information found.
SECTION 4 - FIRST AID MEASURES
Eyes:
Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation.
SECTION 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 1878-49-5: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 155 - 157 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H10O3
Molecular Weight: 166.18
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 1878-49-5: AJ7573000
LD50/LC50:
Not available.
Carcinogenicity:
(2-Methylphenoxy)acetic acid - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 Wear suitable gloves and eye/face protection. WGK (Water Danger/Protection) CAS# 1878-49-5: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 1878-49-5 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 1878-49-5 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 4/03/2003 Revision #2 Date: 12/07/2004 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 2-methylphenoxy acetate 2989-17-5 C10H12O3 180.203 2-甲基苯氧基醋酸乙酯 ethyl (2-methylphenoxy)acetate 93917-68-1 C11H14O3 194.23 2-(2-甲基苯氧基)乙醇 2-methylphenoxyethanol 6161-86-0 C9H12O2 152.193 2-甲基-4-氯苯氧乙酸 MCPA 94-74-6 C9H9ClO3 200.622 —— ortho-methylphenoxyacetamide 22560-43-6 C9H11NO2 165.192 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 2-methylphenoxy acetate 2989-17-5 C10H12O3 180.203 2-(4-羟基-2-甲基苯氧基)乙酸 4-hydroxy-2-methylphenoxyacetic acid 162922-16-9 C9H10O4 182.176 2-甲基苯氧基醋酸乙酯 ethyl (2-methylphenoxy)acetate 93917-68-1 C11H14O3 194.23 2-(2-甲基苯氧基)乙醇 2-methylphenoxyethanol 6161-86-0 C9H12O2 152.193 1-甲基-2-[2-(2-甲基苯氧基)乙氧基]苯 1,2-bis(2-methylphenoxy)ethane 53223-37-3 C16H18O2 242.318 —— o-tolyloxy-acetic acid p-tolyl ester 133192-71-9 C16H16O3 256.301 2-甲基-4-氯苯氧乙酸 MCPA 94-74-6 C9H9ClO3 200.622 2-(4-溴-2-甲基苯氧基)乙酸 4-bromo-2-methylphenoxyacetic acid 6956-82-7 C9H9BrO3 245.073 2-(2-甲基苯氧基)乙酰氯 (2-methylphenoxy)acetyl chloride 15516-43-5 C9H9ClO2 184.622 —— o-tolyloxy-acetic acid m-tolyl ester 133192-70-8 C16H16O3 256.301 —— ortho-methylphenoxyacetamide 22560-43-6 C9H11NO2 165.192 邻甲苯氧基乙腈 (o-tolyloxy)acetonitrile 50635-21-7 C9H9NO 147.177 (4-甲基苯氧基)乙酸 2-(4-methylphenoxy)acetic acid 940-64-7 C9H10O3 166.177 2-(2-甲基苯氧基)乙酰肼 o-tolyloxyacetic acid hydrazide 36304-37-7 C9H12N2O2 180.206 2-氯-6-甲基苯氧基乙酸 2-methyl-6-chlorophenoxyacetic acid 19094-75-8 C9H9ClO3 200.622 —— N-<2-(2-Methylphenoxy)-ethyl>-cyclopropylamin —— C12H17NO 191.273 —— 3-(2-(o-tolyloxy)acetamido)propanoic acid 405923-79-7 C12H15NO4 237.255 2-(2,4-二氯-6-甲基-苯氧基)乙烷酸 (2,4-dichloro-6-methyl-phenoxy)-acetic acid 13333-87-4 C9H8Cl2O3 235.067 2-(2,4-二溴-6-甲基苯氧基)乙酸 (2,4-dibromo-6-methyl-phenoxy)-acetic acid 7250-62-6 C9H8Br2O3 323.969 —— 2-Oxo-2-phenylethyl (2-methylphenoxy)acetate 111922-83-9 C17H16O4 284.312 —— {[4-(chlorosulfonyl)-2-methylphenyl]oxy}acetic acid 23095-23-0 C9H9ClO5S 264.686 —— 2-[(2-Methylphenoxy)methyl]-4,5-dihydro-1,3-oxazole 1063737-87-0 C11H13NO2 191.23 —— N-(2-(piperidin-1-yl)ethyl)-2-(o-tolyloxy)acetamide 1574222-79-9 C16H24N2O2 276.379 —— 4-o-tolyloxyacetyl-morpholine 2021-06-9 C13H17NO3 235.283 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:发现和开发新型苯氧乙酰基酰胺,作为用作冷却剂的高效TRPM8激动剂。摘要:本文介绍了一系列新型的苯氧基乙酰酰胺作为人类TRPM8受体激动剂的活性趋势。该系列涵盖了从微摩尔到皮摩尔水平的体外活性值。这些分子的感官评估突显了它们作为口腔用清凉剂的重要性。对N-(1H-吡唑-3-基)-N-(噻吩-2-基甲基)-2-(对甲苯氧基)乙酰胺进行全面评估的积极结果导致批准了公认的安全(GRAS)调味品和提取物制造商协会(FEMA)的状态为FEMA 4809。DOI:10.1016/j.bmcl.2017.04.003
-
作为产物:描述:参考文献:名称:Aza-Michael Addition of Acrylonitrile with 2-Aryloxymethylbenzimidazole Derivatives under Microwave Irradiation摘要:在微波辐照下,在无水碳酸钾存在下,开发了一种简单、快速、高效的方法,用于丙烯腈与 2-芳基氧甲基苯并咪唑衍生物的偶氮-迈克尔加成反应。制备了一系列新型 1-氰乙基-2-芳氧基甲基苯并咪唑衍生物,并通过 1H NMR、13C NMR、IR 光谱和元素分析进行了表征。DOI:10.3184/030823410x12798039968476
文献信息
-
[EN] PRMT5 INHIBITORS CONTAINING A DIHYDRO- OR TETRAHYDROISOQUINOLINE AND USES THEREOF<br/>[FR] INHIBITEURS DE LA PRMT5 CONTENANT UNE DIHYDRO- OU TÉTRAHYDRO-ISOQUINOLÉINE ET LEURS UTILISATIONS申请人:EPIZYME INC公开号:WO2014100730A1公开(公告)日:2014-06-26Described herein are compounds of Formula (A), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof. Compounds of the present invention are useful for inhibiting PRMT5 activity. Methods of using the compounds for treating PRMT5- mediated disorders are also described.
-
PRMT5 INHIBITORS AND USES THEREOF申请人:Duncan Kenneth W.公开号:US20190083482A1公开(公告)日:2019-03-21Described herein are compounds of Formula (I), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof. Compounds of the present invention are useful for inhibiting PRMT5 activity. Methods of using the compounds for treating PRMT5-mediated disorders are also described.
-
4-((phenoxyalkyl)thio)-phenoxyacetic acids and analogs申请人:DeAngelis Alan公开号:US20060058393A1公开(公告)日:2006-03-16The invention features 4-((phenoxyalkyl)thio)-phenoxyacetic acids and analogs, compositions containing them, and methods of using them as PPAR modulators to treat or inhibit the progression of, for example, dyslipidemia.
-
Sulfamates as antiglaucoma agents申请人:A. H. Robins Company, Incorporated公开号:US05192785A1公开(公告)日:1993-03-09Sulfamate esters of the formula (HO).sub.p --A--[OSO.sub.2 NR.sup.1 R.sup.2 ].sub.z where A is aryloxyalkyl, p is the number of unreacted hydroxy groups present on the alkyl moiety and may be zero, z is the number of --OS(O).sub.2 NR.sup.1 R.sup.2 groups attached to carbons of the alkyl moiety and is always at least one; R.sup.1 and R.sup.2 are selected from hydrogen, loweralkyl, carboxy, and the like are useful in treating glaucoma.Sulfamate酯的化学式为(HO).sub.p --A--[OSO.sub.2 NR.sup.1 R.sup.2 ].sub.z,其中A为芳基氧烷基,p为烷基部分上存在的未反应羟基的数量,可以为零,z为连接到烷基部分碳上的--OS(O).sub.2 NR.sup.1 R.sup.2基团的数量,始终至少为一;R.sup.1和R.sup.2从氢、低烷基、羧基等中选择,在治疗青光眼方面是有用的。
-
Synthesis and in-vitro antimicrobial activity of new 1,2,4-triazoles作者:A R Bhat、G Varadaraj Bhat、G Gautham ShenoyDOI:10.1211/0022357011775307日期:2010.2.18described the synthesis of new 1,2,4-triazoles and have evaluated their antimicrobial profile. Antitubercular activity was determined in triplicate using the Lowenstein-Jensen medium. A loopful of Mycobacterium tuberculosis suspension was inoculated on the surface of each Lowenstein-Jensen media containing the test compounds (100, 10 or 1 microg mL(-1)). To evaluate in-vitro antibacterial activity, compounds我们已经描述了新的1,2,4-三唑的合成,并评估了它们的抗菌特性。使用Lowenstein-Jensen培养基一式三份确定抗结核活性。在含有测试化合物(100、10或1微克mL(-1))的每种Lowenstein-Jensen培养基的表面上接种一圈结核分枝杆菌悬液。为了评估体外抗菌活性,通过圆盘扩散法对枯草芽孢杆菌,大肠杆菌,铜绿假单胞菌,金黄色葡萄球菌和伤寒葡萄球菌评估了化合物(50、5或0.5微克)。为了评估抗真菌活性,使用了Sabourauds葡萄糖琼脂培养基。筛选了某些化合物(5、0.5或0.05微克mL(-1))的抗黑曲霉88和黑曲霉90的活性,而其他化合物则抗T的活性。使用杯板法将红花TR1,红花R.R6,红花R7和薄荷茶T. 我们的结果表明,与在分子4位具有吡嗪部分的三唑相比,在3位具有吡嗪部分的三唑作为抗结核剂和抗真菌剂更具活性。
表征谱图
-
氢谱1HNMR
-
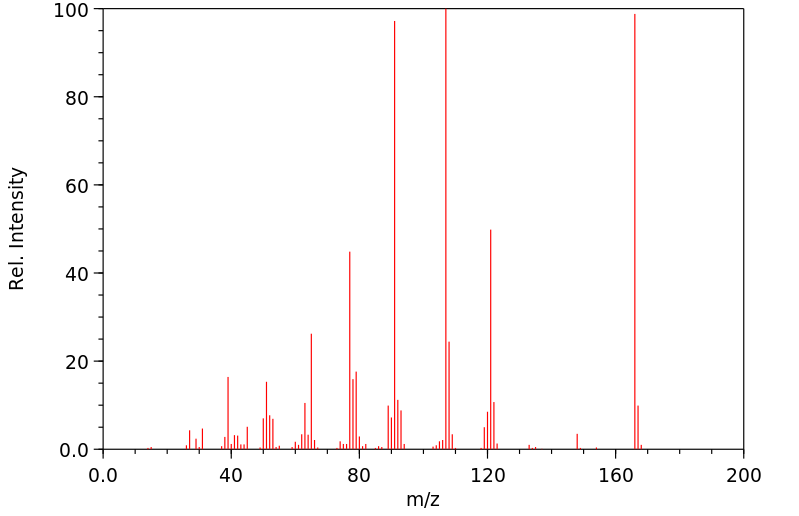
质谱MS
-
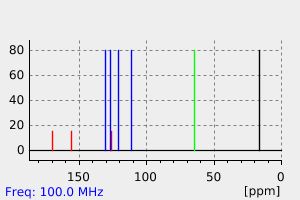
碳谱13CNMR
-
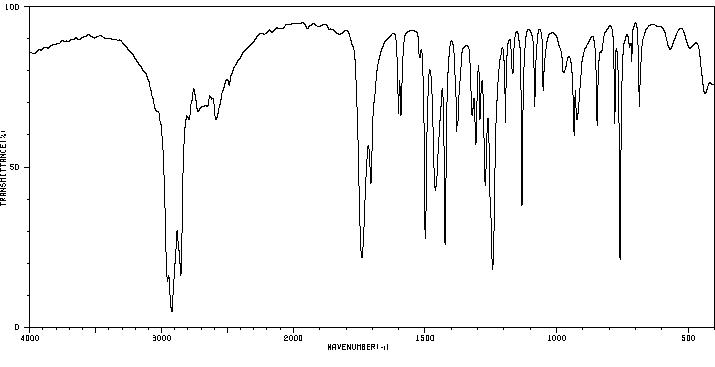
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫