2-萘甲腈 | 613-46-7
中文名称
2-萘甲腈
中文别名
2-氰基萘;萘-2-甲腈;2-氰基萘,萘-2-甲腈;萘-2-腈;2-萘腈
英文名称
2-Cyanonaphthalene
英文别名
2-naphthalenecarbonitrile;2-naphthonitrile;naphthalene-2-carbonitrile
CAS
613-46-7
化学式
C11H7N
mdl
MFCD00016807
分子量
153.183
InChiKey
AZKDTTQQTKDXLH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:63-68 °C
-
沸点:156-158°C 12mm
-
密度:1.0755
-
闪点:156-158°C/12mm
-
溶解度:溶于苯、甲醇
-
保留指数:1497.4;1529;261.3;261.5;255.6;260.88
-
稳定性/保质期:
- 在常温常压下,它不会分解。
- 它存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:Xn,T,N
-
安全说明:S26,S36/37,S36/37/39,S39,S45,S61
-
危险类别码:R20/21/22,R37/38,R51/53,R25,R41
-
WGK Germany:3
-
海关编码:29269090
-
危险品运输编号:3276
-
危险类别:6.1
-
RTECS号:QL5976350
-
包装等级:III
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:贮存: 将密器密封,并储存在密封的主藏器中。应将其置于阴凉、干燥的地方。
SDS
| Name: | 2-Naphthonitrile 97% Material Safety Data Sheet |
| Synonym: | 2-Naphthalenecarbonitrile; beta-Cyanonaphthalene |
| CAS: | 613-46-7 |
Synonym:2-Naphthalenecarbonitrile; beta-Cyanonaphthalene
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 613-46-7 | 2-Naphthonitrile | 97.0 | 210-344-5 |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
The toxicological properties of this substance have not been fully investigated. Metabolism may release cyanide, which may result in headache, dizziness, weakness, collapse, unconsciousness and possible death.
Inhalation:
Harmful if inhaled. May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be metabolized to cyanide which in turns act by inhibiting cytochrome oxidase impairing cellular respiration.
Chronic:
Chronic exposure to cyanide solutions may lead to the development of a "cyanide" rash, characterized by itching, and by macular, papular, and vesicular eruptions, and may be accompanied by secondary infections. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Avoid contact with skin and eyes. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 613-46-7: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: off-white to light beige
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 63-68 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C11H7N
Molecular Weight: 153.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 613-46-7: QL5976350 LD50/LC50:
Not available.
Carcinogenicity:
2-Naphthonitrile - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 613-46-7: No information available.
Canada
CAS# 613-46-7 is listed on Canada's NDSL List.
CAS# 613-46-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 613-46-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
简介
2-萘甲腈是合成一种具有适中介电各向异性的优良液晶材料的重要中间体。此外,许多天然产物和临床药物中都含有氰基团,例如抗肿瘤药Letrozole、β抑制剂Bucindolol、激酶抑制剂Neratinib、心血管药cromakalim、抗HIV药Etravirine、抗痛风药Febuxostat、抗抑郁药Citalopram以及糖尿病药物alogliptin等。
应用领域2-萘甲腈作为有机化学中优良的反应中间体,已被广泛应用于生物学、医药、农药及功能材料等领域中。
制备2-萘甲腈的制备如下:
在充满氮气的手套箱中,向具有封口塞的小瓶中依次加入NiCl₂·6H₂O (5.9 mg, 0.025 mmol),dppf (16.6 mg, 0.03 mmol),Zn (6.5 mg, 0.1 mmol),Zn(CN)₂ (47.0 mg, 0.4 mmol),DMAP (91.6 mg, 0.75 mmol),2-萘甲磺酸酯 (0.5 mmol),CH₃CN (5 mL)。盖好盖子后移出手套箱,直接置于50℃油浴中加热反应8小时。冷却至室温后,经TLC检测并硅胶过滤、乙酸乙酯洗涤、浓缩和柱层析纯化(使用石油醚/乙酸乙酯 = 35:1作为淋洗剂),最终得到白色固体2-萘甲腈71.6 mg,收率93%。经¹HNMR测定纯度大于98%。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-氰基-2-萘酚 6-cyano-2-naphthol 52927-22-7 C11H7NO 169.183 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 —— 2-naphthalenemethanimine —— C11H9N 155.199 2-萘甲醛 β-naphthaldehyde 66-99-9 C11H8O 156.184 2-萘甲醇 2-Naphthalenemethanol 1592-38-7 C11H10O 158.2 2-(氯甲基)萘 2-chloromethylnaphthalene 2506-41-4 C11H9Cl 176.645 2-溴甲基萘 2-bromomethylnaphthyl bromide 939-26-4 C11H9Br 221.096 萘-2-甲胺 naphthalen-2-ylmethylamine 2018-90-8 C11H11N 157.215 2-乙烯基萘 2-Vinylnaphthalene 827-54-3 C12H10 154.211 2-乙基萘 2-ethylnaphthalene 939-27-5 C12H12 156.227 4-溴萘-2-甲腈 4-bromo-2-naphthonitrile 496835-91-7 C11H6BrN 232.079 2-萘乙腈 2-naphthaleneacetonitrile 7498-57-9 C12H9N 167.21 —— 2-naphthaldehyde hydrazone 24091-03-0 C11H10N2 170.214 2-萘基甲基甲基醚 2-(methoxymethyl)naphthalene 42101-92-8 C12H12O 172.227 —— 2-naphthaldoxime 24091-02-9 C11H9NO 171.199 —— (Z)-2-naphthaldoxime —— C11H9NO 171.199 —— (E)-2-naphthaldehyde oxime 51873-98-4 C11H9NO 171.199 苯甲腈 benzonitrile 100-47-0 C7H5N 103.123 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 5-氯萘-2-甲腈 5-chloro-[2]naphthonitrile 73399-86-7 C11H6ClN 187.628 5-溴萘-2-甲腈 5-bromo-2-naphthonitrile 556107-64-3 C11H6BrN 232.079 2-萘甲醛 β-naphthaldehyde 66-99-9 C11H8O 156.184 2-萘甲醇 2-Naphthalenemethanol 1592-38-7 C11H10O 158.2 2-(氯甲基)萘 2-chloromethylnaphthalene 2506-41-4 C11H9Cl 176.645 萘-2-甲胺 naphthalen-2-ylmethylamine 2018-90-8 C11H11N 157.215 —— 2-naphthaldehyde hydrazone 24091-03-0 C11H10N2 170.214 苯甲腈 benzonitrile 100-47-0 C7H5N 103.123
反应信息
-
作为反应物:描述:2-萘甲腈 在 甲基锂 、 双(三甲基硅烷基)氨基钾 作用下, 以 四氢呋喃 、 1,4-二氧六环 为溶剂, 反应 19.58h, 生成 1-(3,4-dihydroquinolin-1(2H)-yl)-2-naphthonitrile参考文献:名称:芳烷基硫醚的亲核胺化和醚化摘要:报道了能够合成二芳基苯胺衍生物的无过渡金属的方案。该方法可以进一步用于制备芳基烷基醚。由于反应条件温和,因此可以耐受多种硫醚,苯胺和醇类。通过进行克级反应(20 mmol),然后将腈基转化为可合成使用的醛,酮和羧酸,证明了我们方法的优势。DOI:10.1021/acs.orglett.8b01758
-
作为产物:参考文献:名称:N,N-二取代酰胺和异丙基酯直接转化为腈摘要:通过用氢化二异丁基铝处理,然后用氨水中的分子碘处理,可以将各种N,N-二甲基酰胺,N-甲氧基-N-甲基酰胺和异丙基酯以良好至中等的收率平稳地转化为相应的腈。本反应是新颖的一锅法和实用方法,其通过分别形成半胱氨酸O -AlBu 2和半缩醛O -AlBu 2来将N,N-二取代酰胺和异丙酯转化为腈。DOI:10.1016/j.tet.2011.04.008
-
作为试剂:描述:参考文献:名称:Enemaerke, Rasmus J.; Christensen, Torben B.; Jensen, Henrik, Journal of the Chemical Society. Perkin Transactions 2 (2001), 2001, # 9, p. 1620 - 1630摘要:DOI:
文献信息
-
Dehydrogenation of Primary Alkyl Azides to Nitriles Catalyzed by Pincer Iridium/Ruthenium Complexes作者:Lan Gan、Xiangqing Jia、Huaquan Fang、Guixia Liu、Zheng HuangDOI:10.1002/cctc.202000260日期:2020.7.21Pincer metal complexes exhibit superior catalytic activity in the dehydrogenation of plain alkanes, but find limited application in the dehydrogenation of functionalized organic molecules. Starting from easily accessible primary alkyl azides, here we report an efficient dehydrogenation of azides to nitriles using pincer iridium or ruthenium complexes as the catalysts. This method offers a route to
-
Nickel-Catalyzed Reversible Functional Group Metathesis between Aryl Nitriles and Aryl Thioethers作者:Tristan Delcaillau、Philip Boehm、Bill MorandiDOI:10.1021/jacs.1c00529日期:2021.3.17We describe a new functional group metathesis between aryl nitriles and aryl thioethers. The catalytic system nickel/dcype is essential to achieve this fully reversible transformation in good to excellent yields. Furthermore, the cyanide- and thiol-free reaction shows high functional group tolerance and great efficiency for the late-stage derivatization of commercial molecules. Finally, synthetic applications
-
A mild and efficient method for the conversion of aldehydes into nitriles and thiols into disulfides using an ionic liquid oxidant作者:Rahman Hosseinzadeh、Hamid Golchoubian、Mahboobe NouzarianDOI:10.1007/s11164-014-1562-4日期:2015.7mild and high yielding method for the conversion of various aldehydes to nitriles has been developed using an ionic liquid reagent, hexamethylene bis( N -methylimidazolium) bis(dichloroiodate) (HMBMIBDCI), in combination with aqueous ammonia in CH3CN at room temperature. Moreover, the treatment of aromatic and aliphatic thiols with HMBMIBDCI resulted in the corresponding disulfides in solvent-free condition
-
Sequential C–H activation enabled expedient delivery of polyfunctional arenes作者:Wensen Ouyang、Xiaoqing Cai、Xiaojian Chen、Jie Wang、Jianhang Rao、Yang Gao、Yanping Huo、Qian Chen、Xianwei LiDOI:10.1039/d1cc03243g日期:——Modular construction of polyfunctional arenes from abundant feedstocks stands as an unremitting pursue in synthetic chemistry, accelerating the discovery of drugs and materials. Herein, using the multiple C–H activation strategy with versatile imidate esters, the expedient delivery of molecular libraries of densely functionalized sulfur-containing arenes was achieved, which enabled the concise construction
-
An Efficient Synthesis of Nitriles from Aldoximes in the Presence of Trifluoromethanesulfonic Anhydride in Mild Conditions作者:N. UludagDOI:10.1134/s1070428020090225日期:2020.9Abstract A new and convenient protocol has been proposed for the transformation of aldoximes to nitriles using trifluoromethanesulfonic anhydride and triethylamine. The proposed method allows a range of aldoximes, including aromatic, heterocyclic, aliphatic, and cycloaliphatic aldoximes, to be converted to the corresponding nitriles in good to excellent yields.
表征谱图
-
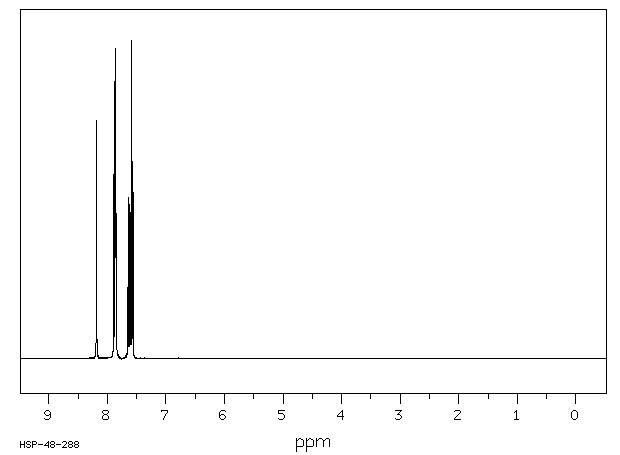
氢谱1HNMR
-
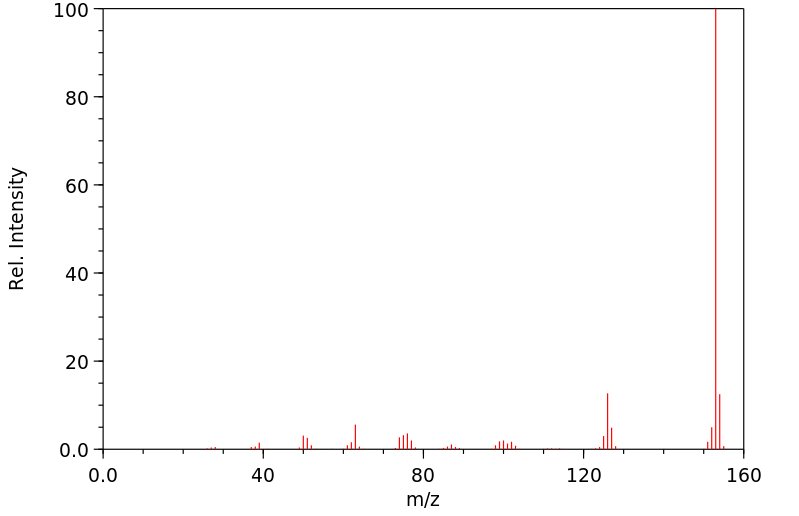
质谱MS
-
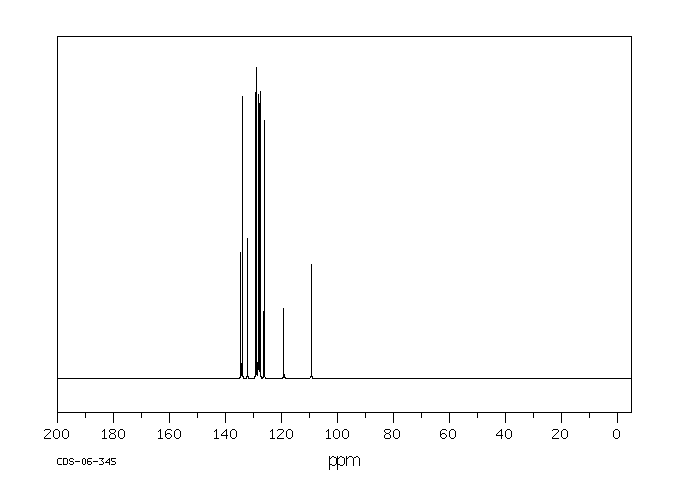
碳谱13CNMR
-
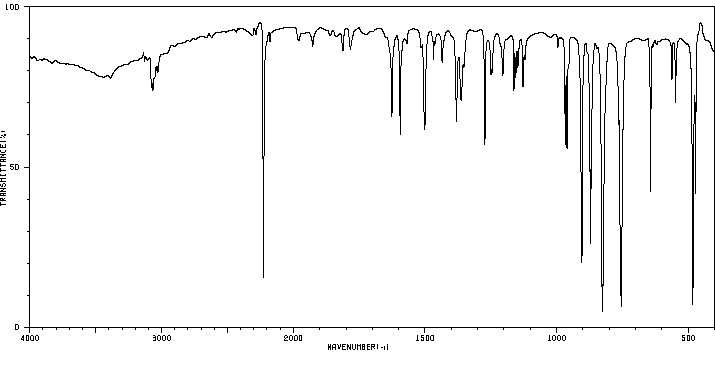
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮