3,3,6-三甲基庚-1,5-二烯-4-醇 | 27644-04-8
中文名称
3,3,6-三甲基庚-1,5-二烯-4-醇
中文别名
——
英文名称
(+-)-artemisia alcohol
英文别名
3,3,6-trimethyl-1,5-heptadien-4-ol;3,3,6-trimethylhepta-1,5-dien-4-ol;Artemisia-alkohol
CAS
27644-04-8
化学式
C10H18O
mdl
MFCD30076501
分子量
154.252
InChiKey
WPPVSYVQAKQNJK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:214.3±9.0 °C(Predicted)
-
密度:0.856±0.06 g/cm3(Predicted)
-
保留指数:1068;1071.8;1073;1069;1073;1070;1082;1069;1076;1068;1066;1074;1074;1074;1076;1065;1065;1064;1074
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:11
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
反应信息
-
作为反应物:描述:参考文献:名称:寻常蒿中的新不规则单萜†摘要:气相色谱研究了艾蒿药草的蒸汽蒸馏油,可鉴定出21种非头尾类异戊二烯骨架的不规则单萜。讨论了其中一些化合物的光谱数据。给出了八个新的不规则单萜的结构。DOI:10.1002/hlca.19810640519
-
作为产物:描述:参考文献:名称:阴离子重排:烯丙基醚阴离子分解和合成应用机理的双重性摘要:DOI:10.1016/0040-4039(70)80082-x
文献信息
-
Highly reactive metals from potassium—graphite. Preparation and use of titanium—graphite and tin—graphite作者:Gian Paolo Boldrini、Diego Savoia、Emilio Tagliavini、Claudio Trombini、Achille Umani-RonchiDOI:10.1016/0022-328x(85)88107-9日期:1985.2The potassium—graphite route to active forms of metals has been extended to the preparation of titanium—graphite (TiGr) and tin—graphite (SnGr). The TiGr is used to achieve the reductive coupling of ketones to give alkenes, and SnGr is used in the preparation of diallyltin dibromide complexes which react with aldehydes to give homoallylic alcohols.
-
Barbier-type allylation of carbonyl compounds and imines with metallic cadmium作者:Bir Sain、Dipak Prajapati、Jagir S SandhuDOI:10.1016/s0040-4039(00)61288-1日期:1992.8Cadmium mediated allylation of a variety of carbonyl compounds and imines in a Cd/Bu4NBr/THF system afforded excellent yields of the corresponding homoallylic alcohols and amines under very mild reaction conditions.
-
Allylic sulfones as allyl anion equivalents: homoallylic alcohols from metal catalysed reactions of sulfones with aldehydes and ketones作者:Jonathan Clayden、Marc JuliaDOI:10.1039/c39940001905日期:——Reduction of allylic sulfones with diethylzinc, catalysed by palladium(0), gives nucleophilic organometallic species, which react in situ with carbonyl compounds to give homoallylic alcohols in high yield.
-
Highly Selective Carbon-Carbon Bond Forming Reactions Mediated by Chromium(II) Reagents作者:Tamejiro Hiyama、Yoshitaka Okude、Keizo Kimura、Hitosi NozakiDOI:10.1246/bcsj.55.561日期:1982.2to produce unisolable allylchromium species which add efficiently to aldehydes or ketones with high degree of stereo- and chemoselectivity. Particularly, high threo selectivity is observed in the reaction of aldehydes and 1-bromo-2-butene and is ascribed to a chair-like six-membered transition state. Simple reduction of allylic and benzylic halides produces biallyls and bibenzyls, while gem-dibromocyclopropanes
-
Un equivalent synthetique chiral du prenal, le formyl-trimethylenemethane (fer) tricarbonyle. synthese du (r)-(-)-ipsdienol作者:Michel Franck-Neumann、Daniel Martina、Marie-Paule HeitzDOI:10.1016/s0040-4039(00)70649-6日期:——organozinc derivatives to Iron stabilized TMM-alcohols. These are decomplexed, under partial hydrogenation, by photolysis in acetic acid to give allylic and homoallylic isoprenoic alcohols. Starting from the optically active complex (+)-3, this allows a rapid synthesis of (R)-(-)-Ipsdienol of high ee.
表征谱图
-
氢谱1HNMR
-
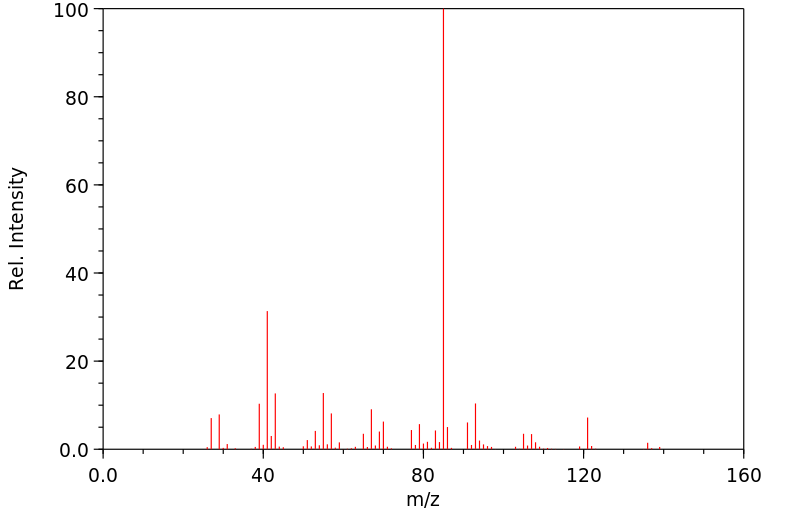
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷