3-乙氧基苯甲醛 | 22924-15-8
中文名称
3-乙氧基苯甲醛
中文别名
间乙氧基苯甲醛
英文名称
3-ethoxybenzaldehyde
英文别名
m-ethoxybenzaldehyde
CAS
22924-15-8
化学式
C9H10O2
mdl
MFCD00016606
分子量
150.177
InChiKey
QZMGMXBYJZVAJN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:177 °C
-
沸点:243 °C(lit.)
-
密度:1.07 g/mL at 25 °C(lit.)
-
闪点:132-134°C/15mm
-
LogP:2.180 (est)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。应避免接触氧化物、碱以及空气中的还原剂。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT, AIR SENSITIVE
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2912499000
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:请将贮藏器密封,并将其放入一个紧密封装的容器中。储存时应选择阴凉、干燥的地方。
SDS
| Name: | 3-Ethoxybenzaldehyde Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 22924-15-8 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 22924-15-8 | 3-Ethoxybenzaldehyde | 98 | 245-333-4 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled. Can produce delayed pulmonary edema.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 22924-15-8: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 243 deg C @ 760 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.07
Molecular Formula: C9H10O2
Molecular Weight: 150.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
None reported.
Incompatibilities with Other Materials:
Strong bases, strong oxidizing agents, strong reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 22924-15-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Ethoxybenzaldehyde - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 22924-15-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 22924-15-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 22924-15-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质:淡黄色的油状液体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-乙氧基-3-甲基苯 3-ethoxytoluene 621-32-9 C9H12O 136.194 间羟基苯甲醛 meta-hydroxybenzaldehyde 100-83-4 C7H6O2 122.123 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-乙氧基苯甲酸 3-ethoxybenzoic acid 621-51-2 C9H10O3 166.177 3-乙氧基苯乙酮 3'-ethoxyacetophenone 52600-91-6 C10H12O2 164.204 甲氧苄醇 3-ethoxy-benzyl alcohol 71648-21-0 C9H12O2 152.193 1-乙烯基-3-乙氧基苯 3-ethoxystyrene 107830-68-2 C10H12O 148.205 1-(氯甲基)-3-乙氧基苯 3-ethoxybenzyl chloride 110207-92-6 C9H11ClO 170.639 3-乙氧基苯甲腈 3-(ethyloxy)benzonitrile 25117-75-3 C9H9NO 147.177 —— 2-(3-Ethoxyphenyl)acetaldehyde —— C10H12O2 164.204 —— 3-ethoxybenzaldoxime 90943-37-6 C9H11NO2 165.192 —— 3-propylethoxybenzene 101144-93-8 C11H16O 164.247 (3-乙氧基苯基)-N-甲基甲胺 (3-Ethoxyphenyl)-N-methylmethanamine 893581-62-9 C10H15NO 165.235 —— 3-(3-Ethoxyphenyl)propanal 1082585-07-6 C11H14O2 178.231 反-3-乙氧基肉桂酸 (E)-3-ethoxycinnamic acid 103986-73-8 C11H12O3 192.214 —— (Z)-3-(3-ethoxyphenyl)acrylic acid 1499179-09-7 C11H12O3 192.214 —— 3-ethoxycinnamic acid 103986-73-8 C11H12O3 192.214 - 1
- 2
反应信息
-
作为反应物:描述:3-乙氧基苯甲醛 在 (S)-联萘(3,5-二甲苯基)膦 、 bis(1,5-cyclooctadiene)iridium(I) tetrakis[3,5-bis(trifluoromethyl)phenyl]borate 作用下, 以 四氢呋喃 为溶剂, 反应 24.0h, 以59%的产率得到苯乙醚参考文献:名称:阳离子铱催化剂通过醛C-H键裂解脱羰摘要:我们报告了通过阳离子铱/双膦催化剂催化的醛 C-H 键裂解使醛脱羰。该反应在相对温和的条件下进行,以中等至高产率得到相应的烃产物。此外,这种阳离子铱催化剂体系可用于酮的不对称加氢酰化。DOI:10.1055/s-0037-1611802
-
作为产物:描述:参考文献:名称:手性N,N-二取代的三氟-3-氨基-2-丙醇是有效的胆固醇酯转移蛋白抑制剂。摘要:描述了可逆地抑制胆固醇酯转移蛋白(CETP)的一系列新的取代的N-苄基-N-苯基-三氟-3-氨基-2-丙醇。从筛选铅22开始,就抑制CETP介导的[(3)H]胆固醇从高密度胆固醇供体颗粒向低密度胆固醇受体颗粒的转移进行了探索,探讨了各种结构特征。丙醇的游离羟基是高效试剂所必需的,因为酰化或烷基化会降低活性。苯胺环中的3-醚部分也具有高抑制效力,而3-苯氧基苯胺类似物表现出最高的效力。活性通过苄基的亚甲基中的氧化或取代而大大降低,这表明苄基环取向对活性很重要。在苄基中,在3-位的取代优于在2-或4-位。在抑制剂中观察到最高的效力,其中3-苄基取代基具有相对于苯环采用平面外取向的潜力。最好的3-苄基取代基是OCF(2)CF(2)H(42,IC(50)缓冲液中0.14 microM,人血清中5.6 microM),环戊基(39),3-异丙氧基(27),SCF (3)(67)和C(CF(3))DOI:10.1021/jm020038h
文献信息
-
Design and synthesis of low molecular weight compounds with complement inhibition activity作者:Hoshang E. Master、Shabana I. Khan、Krishna A. PoojariDOI:10.1016/j.bmc.2005.04.075日期:2005.8An attempt was made to synthesize a series of non-cytotoxic low molecular weight compounds of varying substitutions and functionalities having pharmacophore activity like carbonyl compounds, carboxylic acid and bioisosteres like tetrazole and phenyl acrylic acid. The in vitro assay of these analogues for the inhibition of complement activity revealed significant inhibitory activity for varying substituents
-
[EN] 1,4-DISUBSTITUTED PIPERIDINE DERIVATIVES AND THEIR USE AS 11-BETAHSD1 INHIBITORS<br/>[FR] DERIVES DE PIPERIDINE 1,4 DISUBSTITUEE ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE 11-BETAHSD1申请人:ASTRAZENECA AB公开号:WO2004033427A1公开(公告)日:2004-04-22The use of a compound of formula (I) in the manufacture of a medicament for use in the inhibition of 11βHSD1 is described.使用式(I)的化合物制造用于抑制11βHSD1的药物。
-
A reagent based DOS strategy via Evans chiral auxiliary: highly stereoselective Michael reaction towards optically active quinolizidinones, piperidinones and pyrrolidinones作者:Subhabrata Sen、Siva R. Kamma、Rambabu Gundla、Uma Adepally、Santosh Kuncha、Sridhar Thirnathi、U. Viplava PrasadDOI:10.1039/c2ra22115b日期:——In the present study, we have demonstrated the diversity oriented synthesis of nitrogen heterocycles viz. chiral piperidinones, quinolizidinones and diaryl pyrrolidinones from Michael adducts generated via a TiCl4-catalyzed highly stereoselective Michael reaction with nitrostyrenes and an Evans chiral auxiliary. We also reported a Cu-4,4’-(isopropyl)-substituted isopropylidene-bridged 2,2’-bis-1,3’-oxazoline catalyst mediated catalytic asymmetric version of this reaction. In silico analysis is utilized to evaluate the diversity of the set of compounds against shape space (PMI), polar surface area (PSA) calculations and relevant drug like properties (viz. HBA, HBD, PSA, mol. wt., log P and log D). Finally, the molecules were screened against microorganisms to assess their antimicrobial properties.
-
Novel leucine ureido derivatives as aminopeptidase N inhibitors using click chemistry作者:Jiangying Cao、Chunhua Ma、Jie Zang、Shuai Gao、Qianwen Gao、Xiujie Kong、Yugang Yan、Xuewu Liang、Qin'ge Ding、Chunlong Zhao、Binghe Wang、Wenfang Xu、Yingjie ZhangDOI:10.1016/j.bmc.2018.04.041日期:2018.7is associated with the tumor angiogenesis and metastasis. In this report, one new series of leucine ureido derivatives containing the triazole moiety was designed, synthesized and evaluated as APN inhibitors. Among them, compound 13v showed the best APN inhibition with an IC50 value of 0.089 ± 0.007 μM, which was two orders of magnitude lower than that of bestatin (IC50 = 9.4 ± 0.5 μM). Compound 13v
-
Discovery of Antimetastatic Chiral Ionone Alkaloid Derivatives Targeting HIF‐1α/VEGF/VEGFR2 Pathway作者:Jing‐Jing Liu、Xin‐Yao Liu、Jiang‐Ping Nie、Mei‐Qi Jia、Yang Yu、Nan Qin、Hong‐Quan DuanDOI:10.1002/cmdc.202100072日期:——Novel chiral ionone alkaloid derivatives were synthesized and their antimetastatic effects were evaluated in human breast cancer cells using chemotaxis assay. Compared with positive control LY294002, a PI3 K inhibitor, derivatives 10 a, 11 a, 11 c, 11 g, 11 j, 11 k and 11 w exhibited significant inhibitory effects against cancer cell migration. Especially, the IC50 for compound 11 g was as low as 0合成了新型手性紫罗兰酮生物碱衍生物,并使用趋化性测定在人乳腺癌细胞中评估了它们的抗转移作用。与阳性对照 LY294002 相比,PI3 K 抑制剂衍生物 10a 、11a、11c、11g、11j、11k和11w对癌细胞迁移具有显着的抑制作用。特别是化合物11 g的IC 50低至0.035±0.004 μM。对化合物11 g的进一步研究表明,它对 MDA-MB-231 细胞的粘附、迁移和侵袭具有抑制作用。11g抗肿瘤转移作用的机制可能是通过抑制 HIF-1α/VEGF/VEGFR2/Akt 通路,从而抑制下游信号分子,包括 Akt1/mTOR/p70S6K 和 Akt2/PKCζ/整合素 β1 通路。综上所述,手性紫罗兰酮生物碱衍生物11 g具有开发成为乳腺癌抗肿瘤转移剂的潜力。
表征谱图
-
氢谱1HNMR
-
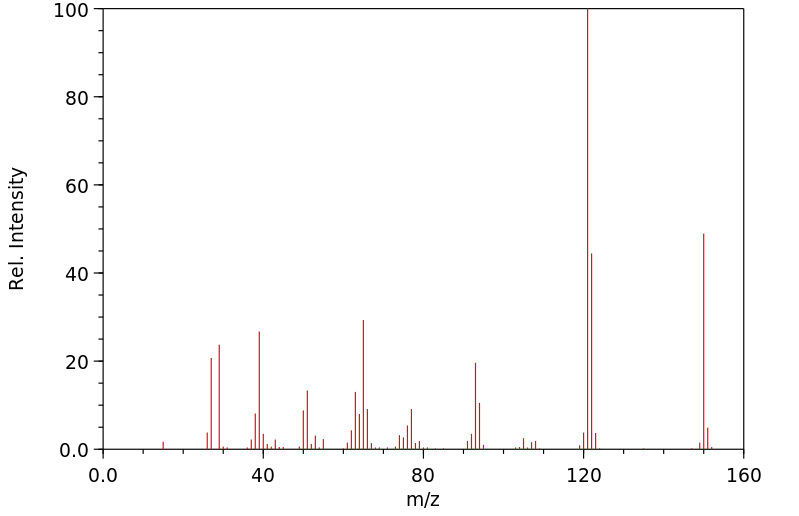
质谱MS
-
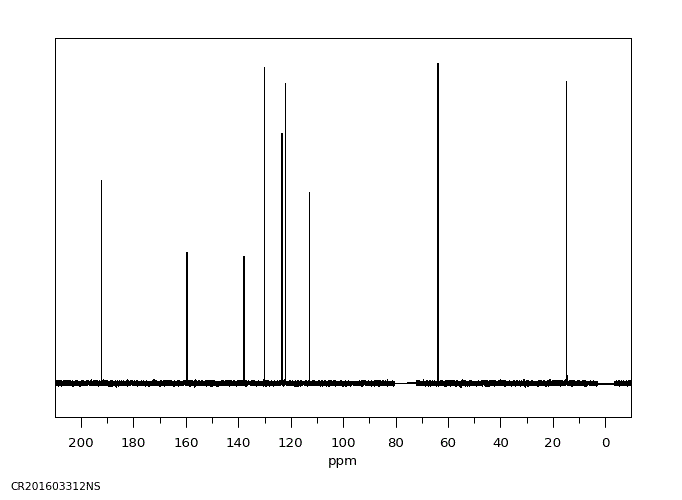
碳谱13CNMR
-
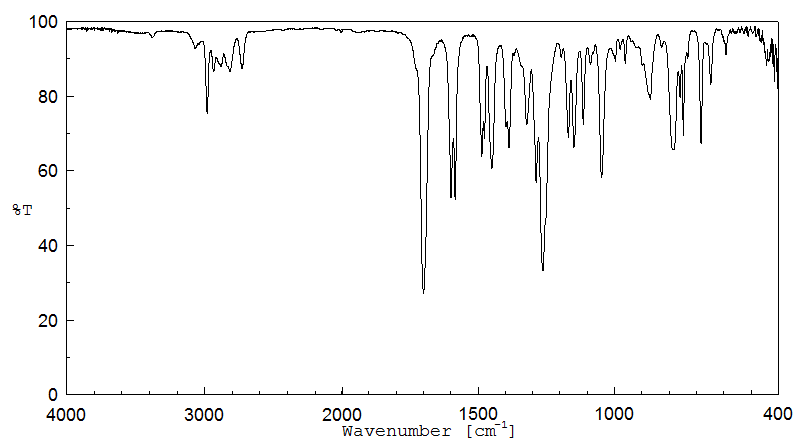
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯