3-异丙氧基丙腈 | 110-47-4
中文名称
3-异丙氧基丙腈
中文别名
——
英文名称
3-(propan-2-yloxy)propanonitrile
英文别名
3-isopropoxypropanenitrile;3-propan-2-yloxypropanenitrile
CAS
110-47-4
化学式
C6H11NO
mdl
MFCD00019866
分子量
113.159
InChiKey
BMSYXLRQGIFLFO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:179℃
-
密度:0.8969 g/cm3
-
保留指数:879
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:33
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
海关编码:2926909090
SDS
| Name: | Isopropoxypropionitrile 99+% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 110-47-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 110-47-4 | 3-isopropoxypropionitrile | 99+ | 203-771-3 |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Appearance: clear, colorless.
Not available.
Target Organs: None.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits +--------------------+-------------------+-------------------+-----------------+ | Chemical Name | ACGIH | NIOSH |OSHA - Final PELs| |--------------------|-------------------|-------------------|-----------------| | 3-isopropoxypropion|none listed |none listed |none listed | | itrile | | | | +--------------------+-------------------+-------------------+-----------------+ OSHA Vacated PELs: 3-isopropoxypropionitrile: No OSHA Vacated PELs are listed for this chemical.
Personal Protective Equipment Eyes: Wear chemical goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant a respirator's use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Appearance: clear, colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 0 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
NFPA Rating: health-1; flammability-2; reactivity-1
Explosion Limits, Lower: Not available.
Upper: Not available.
Decomposition Temperature:
Solubility:
Specific Gravity/Density:
Molecular Formula: C6H11NO
Molecular Weight: 113.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 110-47-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-isopropoxypropionitrile - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Chemical waste generators must determine whether a discarded chemical is classif as a hazardous waste.
US EPA guidelines for the classification determination are listed in 40 CFR Part Additionally, waste generators must consult state and local hazardous waste regu ensure complete and accurate classification.
RCRA P-Series: None listed.
RCRA U-Series: None listed.
Section 14 - TRANSPORT INFORMATION
CDG/CPL
Not classified as hazardous for supply.
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 110-47-4: 2
United Kingdom Occupational Exposure Limits
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
WHMIS: Not available.
CAS# 110-47-4 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 110-47-4 is listed on the TSCA inventory.
Health & Safety Reporting List
None of the chemicals are on the Health & Safety Reporting List.
Chemical Test Rules
None of the chemicals in this product are under a Chemical Test Rule.
Section 12b
None of the chemicals are listed under TSCA Section 12b.
TSCA Significant New Use Rule
None of the chemicals in this material have a SNUR under TSCA.
SARA
Section 302 (RQ)
None of the chemicals in this material have an RQ.
Section 302 (TPQ)
None of the chemicals in this product have a TPQ.
Section 313
No chemicals are reportable under Section 313.
Clean Air Act:
This material does not contain any hazardous air pollutants.
This material does not contain any Class 1 Ozone depletors.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:The Cleavage of β-Oxypropionitriles with Lithium Aluminum Hydride1摘要:DOI:10.1021/ja01642a067
-
作为产物:描述:3-异丙氧基丙胺 在 copper(l) iodide 、 氧气 作用下, 以 二氯甲烷 为溶剂, 20.0 ℃ 、101.33 kPa 条件下, 反应 6.0h, 以88%的产率得到3-异丙氧基丙腈参考文献:名称:胺的需氧氧化的简单铜催化剂:抗衡离子的选择性控制摘要:我们描述了在将胺选择性好氧氧化为腈或亚胺时,使用简单的铜盐催化剂的方法。这些催化剂以其出色的效率而著称,可在环境温度和压力下运行,并且无需昂贵的配体或添加剂即可氧化胺。这项研究强调了抗衡离子在控制好氧氧化中的选择性方面可以发挥重要作用。DOI:10.1002/anie.201609255
文献信息
-
KO <i>t</i> Bu‐Catalyzed Michael Addition Reactions Under Mild and Solvent‐Free Conditions作者:Subramanian Thiyagarajan、Varadhan Krishnakumar、Chidambaram GunanathanDOI:10.1002/asia.201901647日期:2020.2.17predominantly catalyze Michael addition reactions. Inorganic and organic base‐catalyzed Michael addition reactions have been reported. However, known base‐catalyzed reactions suffer from the requirement of solvents, additives, high pressure and also side‐reactions. Herein, we demonstrate a mild and environmentally friendly strategy of readily available KOtBu‐catalyzed Michael addition reactions. This simple
-
Novel amine-catalysed hydroalkoxylation reactions of activated alkenes and alkynes作者:Julie E. Murtagh、S�amus H. McCooey、Stephen J. ConnonDOI:10.1039/b414895a日期:——Substoichiometric loadings of DBU catalyse the efficient 1,4-addition of alcohols and non-nucleophilic amines such as pyrrole to activated alkenes; the application of this methodology in a one-pot synthesis of a natural product, and as a novel strategy for the synthesis of mono-protected 1,3-carbonyl compounds is reported.
-
Formation of a New, Strongly Basic Nitrogen Anion by Metal Oxide Modification作者:Masazumi Tamura、Ryota Kishi、Akira Nakayama、Yoshinao Nakagawa、Jun-ya Hasegawa、Keiichi TomishigeDOI:10.1021/jacs.7b05227日期:2017.8.30viewpoints. Here, a principle for creating a new strong base by hybridization of homogeneous and heterogeneous components is presented. It is based on the modification of organic compounds with metal oxides by using the acid-base property of metal oxides. Based on kinetic and DFT studies, combination of CeO2 and 2-cyanopyridine drastically enhanced the basicity of 2-cyanopyridine by a factor of about 109开发具有独特且前所未有的催化性能的新型杂化材料对化学家来说是一个挑战,而多相-均相杂化催化剂由于期望由组分组合产生的优选和特殊性能而备受关注。碱催化剂作为关键材料广泛应用于有机合成中,一类新的碱催化剂在学术和工业上都产生了很大的影响。在这里,提出了通过同质和异质成分的杂交来创建新的强碱的原理。它是利用金属氧化物的酸碱性质,用金属氧化物对有机化合物进行改性。基于动力学和 DFT 研究,CeO2 和 2-氰基吡啶的组合使 2-氰基吡啶的碱性显着提高了约 109 倍(pKa(在 CH3CN 中)约为 9),估计 pKa 为 21,这将其置于超强碱类别中。2-氰基吡啶和 通过两种相互作用模式形成独特的吸附复合物:(i) 的Ce原子与2-氰基吡啶中吡啶环的N原子之间的配位相互作用,以及(ii)表面O原子之间的共价相互作用通过将 的晶格氧加成到 2-氰基吡啶的 CN 基团上,
-
Effect of pressure on sterically congested cyanoalkylation reactions of alcohols作者:G. JennerDOI:10.1016/s0040-4020(02)00347-2日期:2002.5The pressure effect on the phosphine-catalyzed nucleophilic addition of alcohols to unsaturated nitriles is examined. As a general result, pressure promotes these reactions. Their sensitivity to pressure increases with increasing steric congestion of either the alcohol or the nitrile. Activation volumes are found to be very negative pointing not only to a late transition state, but essentially to a考察了对膦向不饱和腈的膦催化的亲核加成反应的压力影响。通常,压力会促进这些反应。它们对压力的敏感性随着酒精或腈的空间拥塞的增加而增加。发现活化体积是非常负的,不仅指向晚期转变状态,而且还取决于取决于电离的空间位阻,基本上指向相当大的电致伸缩贡献。这意味着压力有利于碳负离子的形成和腈的侵蚀。该结果突出了高压的合成效用,以消除空间抑制作用。
-
Electron-rich triarylphosphines as nucleophilic catalysts for oxa-Michael reactions作者:Susanne M Fischer、Simon Renner、A Daniel Boese、Christian SlugovcDOI:10.3762/bjoc.17.117日期:——and intermediate Michael acceptors such as acrylamide and acrylonitrile and for converting less acidic alcohols like isopropanol. With stronger Michael acceptors and more acidic alcohols, the impact of the more electron-rich catalysts is less pronounced. The experimental activity trend was rationalized by calculating the Michael acceptor affinities of all phosphine–Michael acceptor combinations. Besides富电子三芳基膦,即 4-(甲氧基苯基)二苯基膦 (MMTPP) 和三(4-三甲氧基苯基)膦 (TMTPP) 在催化 oxa-Michael 加成方面优于常用的三苯基膦 (TPP)。使用由三种不同强度的迈克尔受体和四种不同酸度的醇组成的基质来评估三种催化剂的活性。所有测试反应均在无溶剂条件下和室温下以 1 mol% 催化剂负载量进行。结果表明,TMTPP 在转化丙烯酰胺和丙烯腈等弱迈克尔受体和中间迈克尔受体以及转化异丙醇等弱酸性醇方面具有决定性的优势。随着迈克尔受体的增强和酸性醇的增多,富电子催化剂的影响就不那么明显了。通过计算所有膦-迈克尔受体组合的迈克尔受体亲和力,使实验活性趋势合理化。除了这个参数之外,醇的酸度对反应速度也有很大影响。还评估了膦的氧化稳定性,发现最富电子的 TMTPP 对氧化的敏感性仅比 TPP 稍高。最后,将该催化剂用于丙烯酸2-羟乙酯的氧杂-迈克尔聚合反应。使用TM
表征谱图
-
氢谱1HNMR
-
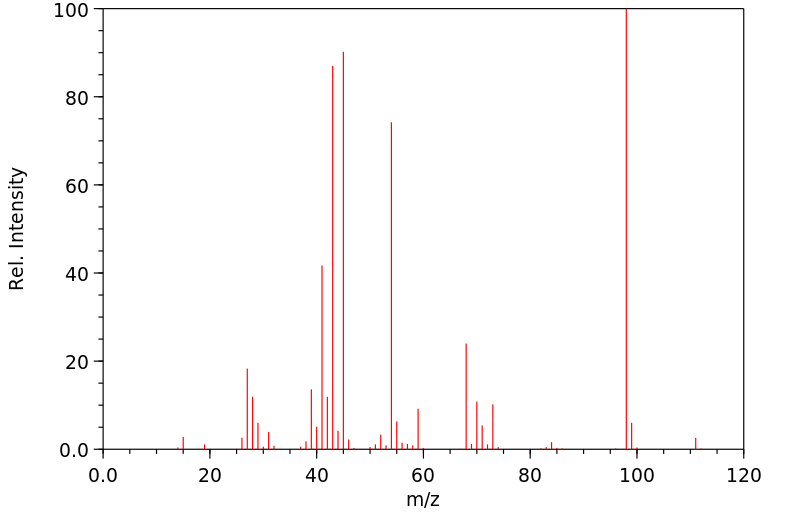
质谱MS
-
碳谱13CNMR
-
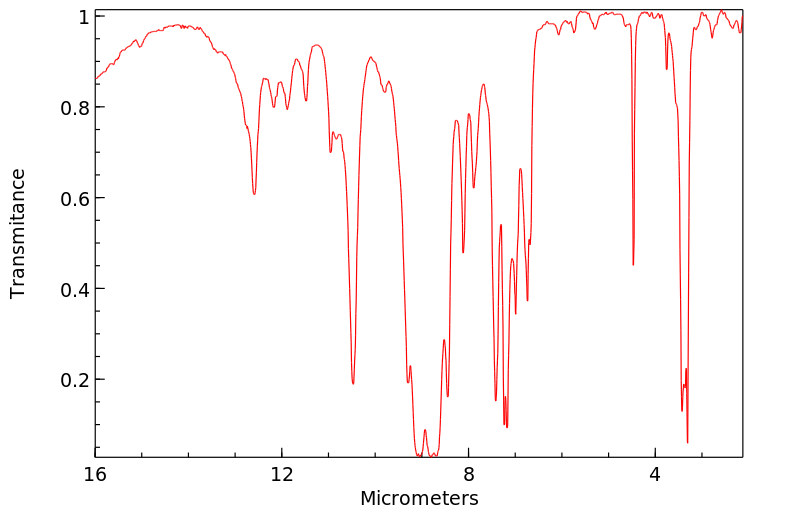
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷