3-氯辛烷 | 1117-79-9
中文名称
3-氯辛烷
中文别名
——
英文名称
3-chlorooctane
英文别名
3-chloro-octane;3-Chlor-octan;ethylchlorohexane;3-Chlor-n-octan;Octylchlorid-3;3-Chloroctan
CAS
1117-79-9
化学式
C8H17Cl
mdl
——
分子量
148.676
InChiKey
FYNJWLOOBINARS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-41.9°C (estimate)
-
沸点:178.66°C (estimate)
-
密度:0.8765 (estimate)
-
保留指数:1006;1006
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903199000
SDS
反应信息
-
作为反应物:参考文献:名称:外消旋 4-乙基脂肪酸的简便合成摘要:报道了外消旋 4-乙基脂肪酸的合成。首先通过 3-氯代烷烃与镁反应制备格氏试剂,然后在催化剂 Li2CuCl4 存在下,通过将格氏试剂与 3-溴丙酸甲酯偶联合成 4-乙基脂肪酸甲酯。将4-乙基脂肪酸甲酯皂化,然后酸化,得到4-乙基脂肪酸。描述了4-乙基己酸、4-乙基庚酸、4-乙基辛酸、4-乙基壬酸和4-乙基癸酸的合成。4-乙基脂肪酸甲酯和4-乙基脂肪酸的结构经1H NMR、13C NMR和HRMS证实。DOI:10.3184/174751912x13402672600994
-
作为产物:描述:参考文献:名称:可回收离子液体 [bmim][X] 磺酸酯亲核取代反应的简便绿色方案摘要:离子液体 [bmim][X] (X = Cl, Br, I, OAc, SCN) 是用于伯醇和仲醇衍生的磺酸酯亲核取代反应的高效试剂。离子液体的抗衡阴离子 (X-) [bmim][X] 有效地替代了磺酸盐,以优异的产率提供了相应的取代产物,如烷基卤化物、乙酸盐和硫氰化物。新开发的协议对环境非常有吸引力,因为在大多数情况下,反应使用化学计量的离子液体作为唯一试剂,不需要额外的溶剂、任何其他活化试剂、非常规设备或特殊预防措施。此外,这些离子液体可以很容易地回收利用而不会损失反应性,从而使整个过程“更环保”。DOI:10.1055/s-0032-1317473
文献信息
-
Reactivity of bismuth(III) halides towards alcohols. A tentative to mechanistic investigation作者:El Mehdi Keramane、Bernard Boyer、Jean-Pierre RoqueDOI:10.1016/s0040-4020(01)00013-8日期:2001.3bismuth(III) halides (BiX3; X=Cl, Br and I) towards a series of alcohols has been investigated. Three different reactions have been studied, namely: halogenation, dehydration and etherification. The behaviour of these bismuth derivatives was found to depend on the nature of the halide bonded to the bismuth atom. Their reactivities can be interpreted on the basis of the Hard and Soft Acids and Bases
-
Stereoretentive Chlorination of Cyclic Alcohols Catalyzed by Titanium(IV) Tetrachloride: Evidence for a Front Side Attack Mechanism作者:Deboprosad Mondal、Song Ye Li、Luca Bellucci、Teodoro Laino、Andrea Tafi、Salvatore Guccione、Salvatore D. LeporeDOI:10.1021/jo3023439日期:2013.3.1A mild chlorination reaction of alcohols was developed using the classical thionyl chloride reagent but with added catalytic titanium(IV) chloride. These reactions proceeded rapidly to afford chlorination products in excellent yields and with preference for retention of configuration. Stereoselectivities were high for a variety of chiral cyclic secondary substrates including sterically hindered systems使用经典的亚硫酰氯试剂开发了醇的温和氯化反应,但添加了催化氯化钛 (IV)。这些反应进行得很快,以极好的收率提供氯化产物,并优先保留构型。包括空间位阻系统在内的各种手性环状次级底物的立体选择性都很高。氯亚硫酸盐首先在原位生成并通过四氯化钛的作用转化为烷基氯化物,四氯化钛被认为螯合氯亚硫酸盐离去基团并从正面释放卤素亲核试剂。为了更好地理解这种新的反应途径,使用两种不同的计算方法在 DFT 理论水平上进行了从头研究。这一计算证据表明,虽然反应通过碳正离子中间体进行,但这种带电物质可能保留金字塔几何形状,作为通过超共轭(超共轭体)稳定的构象异构体而存在。然后,这些碳正离子在亲核捕获时基本上“冻结”为其原始构型。
-
Iron(III)-Catalyzed Halogenations by Substitution of Sulfonate Esters作者:Nuria Ortega、Andrés Feher-Voelger、Margarita Brovetto、Juan I. Padrón、Victor S. Martín、Tomás MartínDOI:10.1002/adsc.201000740日期:2011.4.18A novel halogenation reaction from sulfonates catalyzed by iron(III) is described. The reaction can be performed as a stoichiometric or a catalytic version. This reaction provides a convenient strategy for the efficient access to structurally diverse secondary chlorides, bromides and iodides. The stereochemical course of the reaction is governed by the substrate and the experimental conditions. Secondary
-
Process for preparing monochlorinated hydrocarbons having a high isomeric purity申请人:DEGUSSA AG公开号:US20030065232A1公开(公告)日:2003-04-03Monochlorinated hydrocarbons of high isomeric purity are prepared by a process, which comprises: reacting a monoalcohol having an alkyl radical having from 3 to 20 carbon atoms with cyanuric chloride; and purifying the resulting monochlorinated hydrocarbon by distillation after separation of salts and washing the monochlorinated hydrocarbon with alkali. The invention relates to a process for preparing monochlorinated hydrocarbons which contain an alkyl radical having from 3 to 20 carbon atoms and have a high isomeric purity by reacting a monoalcohol having a hydrocarbon radical containing an alkyl radical having from 3 to 20 carbon atoms to which additional cycloaliphatic radicals, aryl radicals, aralkyl radicals and alkylaryl radicals may be bound with cyanuric chloride, separating off salts, washing the reaction mixture with alkali and purifying the resulting monochlorinated hydrocarbons by distillation.
-
Clarification of the Stereochemical Course of Nucleophilic Substitution of Arylsulfonate-Based Nucleophile Assisting Leaving Groups作者:D. Christopher Braddock、Rebecca H. Pouwer、Jonathan W. Burton、Phillip BroadwithDOI:10.1021/jo900991z日期:2009.8.21Secondary alcohols modified as tosylates, PEG-sulfonates, or quisylates undergo inversion of configuration at the reacting center when treated with lithium halide in acetone at reflux, where the PEG-sulfonates and quisylates are substantially more reactive. In sterically hindered cases, elimination is a competing process. In contrast, when treated with TiCl4, simple secondary sulfonates give chloride
表征谱图
-
氢谱1HNMR
-
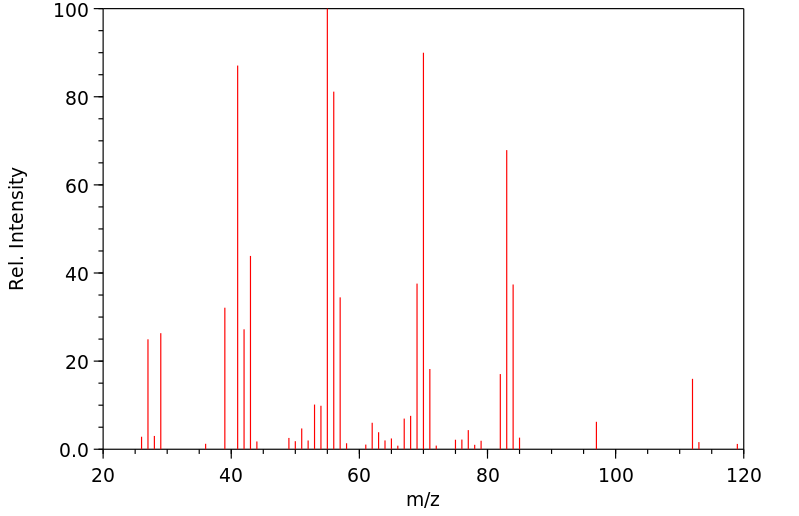
质谱MS
-
碳谱13CNMR
-
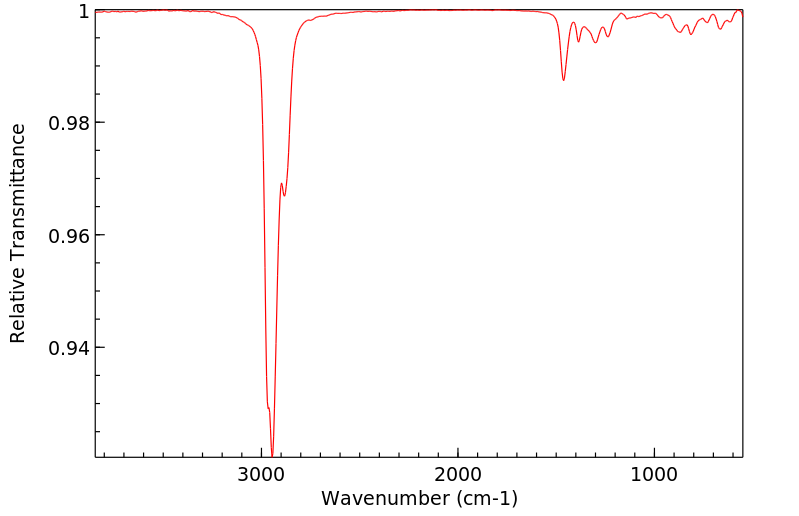
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷