3-甲基-1-壬炔-3-醇 | 5430-01-3
中文名称
3-甲基-1-壬炔-3-醇
中文别名
——
英文名称
3-methyl-1-nonyn-3-ol
英文别名
3-Methyl-nonin-(1)-ol-(3);3-methyl non-1-yn-3-ol;3-methylnon-1-yn-3-ol
CAS
5430-01-3
化学式
C10H18O
mdl
MFCD00041582
分子量
154.252
InChiKey
VQUXVWMAXIQKTQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:87-88°C 15mm
-
密度:0.881±0.06 g/cm3(Predicted)
-
闪点:87-88°C/15mm
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:11
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
海关编码:2905290000
-
安全说明:S24/25
-
储存条件:常温下避光存放于阴凉干燥处,并密封保存。
SDS
上下游信息
反应信息
-
作为反应物:描述:3-甲基-1-壬炔-3-醇 在 copper(l) iodide 、 四甲基乙二胺 、 氧气 作用下, 以 乙腈 为溶剂, 以83%的产率得到7,12-dimethyloctadeca-8,10-diyne-7,12-diol参考文献:名称:1,3-二炔的钯催化键重组:多样化功能化的1,5-二烯-3-炔的进入摘要:描述了在Pd II催化下从1,3-二炔合成功能化的1,5-二烯-3-炔的温和而有效的方法。该方法允许以高收率快速且原子经济地组装各种二卤代,卤代酰基和二酰基取代的1,5-二烯-3-炔。通过选择催化体系和反应条件,可以控制这些二烯炔产物形成过程中的选择性转换。DOI:10.1021/jo400276e
-
作为产物:描述:参考文献:名称:New organoboron reagents for the preparation unsubstituted propargylic摘要:本发明的新型有机硼试剂在制备未取代的丙炔醇中非常有用。该化合物与醛和酮反应干净,可以得到产率极高的丙炔醇。未取代的丙炔醇是合成许多天然产物的重要中间体。此外,本发明的新型有机硼试剂在与对映纯醛反应时还表现出对映选择性。公开号:US05110966A1
文献信息
-
Visible‐Light‐Promoted Regio‐ and Stereoselective Oxyalkenyl‐ation of Phosphinyl Allenes作者:Xue Sun、Teng Liu、Yan‐Tong Yang、Yue‐Jie Gu、Yu‐Wei Liu、Yi‐Gang Ji、Kai Luo、Jie Zhu、Lei WuDOI:10.1002/adsc.202000214日期:2020.7.16time. This protocol, merging visible light photoredox and palladium catalysis, provides a direct approach to conjugated tertiary allylic alcohol derivatives with broad functional group tolerance in moderate to excellent yields. Mechanistic studies suggest that, although two possible pathways exist in the transformation, radical oxyalkenylation promoted by visible light photoredox takes over the major
-
A CO<sub>2</sub>-mediated base catalysis approach for the hydration of triple bonds in ionic liquids作者:Minhao Tang、Fengtao Zhang、Yanfei Zhao、Yuepeng Wang、Zhengang Ke、Ruipeng Li、Wei Zeng、Buxing Han、Zhimin LiuDOI:10.1039/d1gc03865f日期:——approach for the activation of triple bonds in ionic liquids (ILs) with anions that can chemically capture CO2 (e.g., azolate, phenolate, and acetate), which can achieve hydration of triple bonds to carbonyl chemicals. It is discovered that the anion-complexed CO2 could abstract one proton from proton resources (e.g., IL cation) and transfer it to the CN or CC bonds via a six-membered ring transition
-
Alkene Difunctionalization Triggered by a Stabilized Allenyl Radical: Concomitant Installation of Two Unsaturated C−C Bonds作者:Yunlong Wei、Hong Zhang、Xinxin Wu、Chen ZhuDOI:10.1002/anie.202106145日期:2021.9.6Radical-mediated difunctionalization of alkenes provides a promising approach to introduce one alkenyl or alkynyl group to target compounds. However, simultaneous installation of two unsaturated C−C bonds via alkene difunctionalization remains elusive, attributable to the high instability and transient lifetimes of alkenyl and alkynyl radicals. Herein, we report the photocatalytic 1,2-alkynylalkenylation and烯烃的自由基介导的双官能化提供了一种将一个烯基或炔基引入目标化合物的有前途的方法。然而,由于烯基和炔基自由基的高不稳定性和瞬态寿命,通过烯烃双官能化同时安装两个不饱和的 C-C 键仍然难以捉摸。在此,我们首次报告了烯烃的光催化 1,2-炔基烯基化和 1,2- 烯炔基化,这是由稳定的烯基自由基与烯烃的分子间加成引发的。一系列经过战略设计、易于使用的双功能试剂被应用于自由基对接-迁移级联。该协议具有广泛的底物范围,并有效地增加了不饱和度。
-
Thermodynamic favorable CO2 conversion via vicinal diols and propargylic alcohols: A metal-free catalytic method作者:Li-Hua Han、Jing-Yuan Li、Qing-Wen Song、Kan Zhang、Qian-Xia Zhang、Xiao-Fang Sun、Ping LiuDOI:10.1016/j.cclet.2019.06.030日期:2020.2promising field in chemical fixation of CO2. Herein, a facile metal-free strategy was reported for the one-pot preparation of cyclic carbonates and α-hydroxy ketones from vicinal diols, propargylic alcohols and CO2. Wide scope of vicinal diols and propargylic alcohols was demonstrated to be efficient under the DBU-catalyzed conditions. A plausible mechanism was proposed, which included detailed main and side
-
‘Click’ ligand for ‘click’ chemistry: (1-(4-methoxybenzyl)-1-H-1,2,3-triazol-4-yl)methanol (MBHTM) accelerated copper-catalyzed [3+2] azide–alkyne cycloaddition (CuAAC) at low catalyst loading作者:Rajesh H. Tale、Venkatesh B. Gopula、Gopal K. ToradmalDOI:10.1016/j.tetlet.2015.09.010日期:2015.101,2,3-triazol-4-yl)methanol (MBHTM) synthesized itself by ‘click’ chemistry shown to promote the dramatic rate enhancement of copper catalyze azide–alkyne cycloaddition (CuAAC) at low catalyst loading to give diverse 1,4-disustituted 1,2,3-triazoles in high to excellent yields under mild conditions. Using higher catalyst and ligand loading, excellent and 49% yield of the corresponding 1,2,3 triazole
表征谱图
-
氢谱1HNMR
-
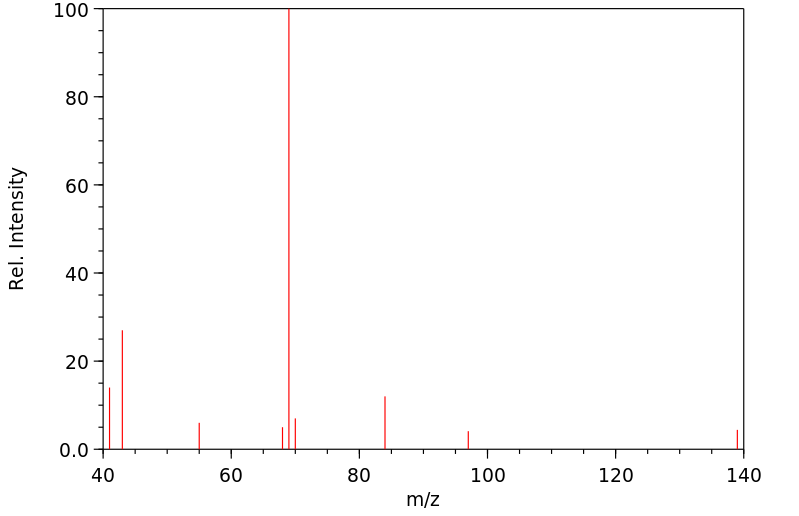
质谱MS
-
碳谱13CNMR
-
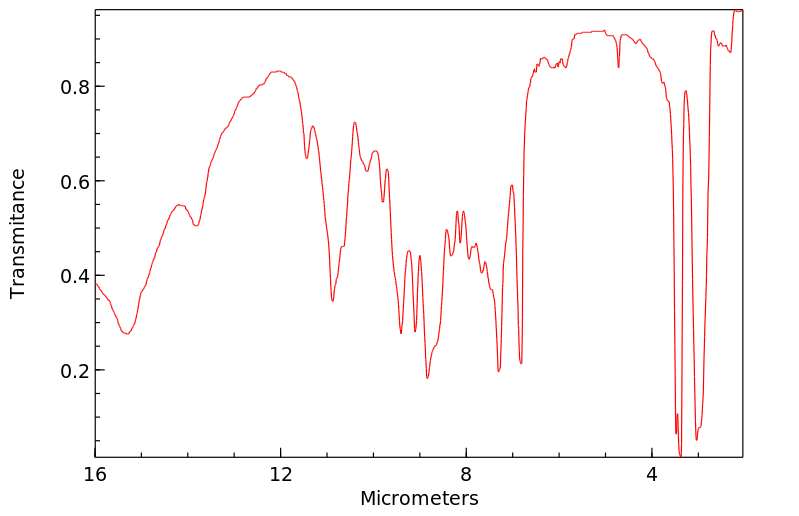
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷