3-甲基邻苯二甲酸酐 | 4792-30-7
中文名称
3-甲基邻苯二甲酸酐
中文别名
4-甲基异苯并呋喃-1,3-二酮
英文名称
3-methylphthalic acid anhydride
英文别名
3-methylphthalic anhydride;4-methylisobenzofuran-1,3-dione;4-methyl-2-benzofuran-1,3-dione
CAS
4792-30-7
化学式
C9H6O3
mdl
MFCD00047316
分子量
162.145
InChiKey
TWWAWPHAOPTQEU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:114-118 °C
-
沸点:248.82°C (rough estimate)
-
密度:1.2599 (rough estimate)
-
保留指数:1396.2;241.23
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.111
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2932999099
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
3-甲基邻苯二甲酸酐 修改号码:5
模块 1. 化学品
产品名称: 3-Methylphthalic Anhydride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-甲基邻苯二甲酸酐
百分比: >96.0%(T)
CAS编码: 4792-30-7
分子式: C9H6O3
3-甲基邻苯二甲酸酐 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
3-甲基邻苯二甲酸酐 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅红黄色
气味: 无资料
pH: 无数据资料
熔点:
118°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
3-甲基邻苯二甲酸酐 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3-Methylphthalic Anhydride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-甲基邻苯二甲酸酐
百分比: >96.0%(T)
CAS编码: 4792-30-7
分子式: C9H6O3
3-甲基邻苯二甲酸酐 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
3-甲基邻苯二甲酸酐 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅红黄色
气味: 无资料
pH: 无数据资料
熔点:
118°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
3-甲基邻苯二甲酸酐 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5,6-二甲基-2-苯并呋喃-1,3-二酮 4,5-dimethylphthalic anhydride 5999-20-2 C10H8O3 176.172 2-甲基-苯甲酸酐 2-methylbenzoic anhydride 607-86-3 C16H14O3 254.285 3-甲基邻苯二甲酸 3-methylphthalic acid 37102-74-2 C9H8O4 180.16 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-甲基-2-苯并呋喃-1(3H)-酮 4-methylphthalide 2211-83-8 C9H8O2 148.161 —— 1,3-dihydro-3-oxo-4-isobenzofurancarboxylic acid 4792-28-3 C9H6O4 178.144 7-甲基苯酞 7-methyl-3H-isobenzofuran-1-one 2211-84-9 C9H8O2 148.161 —— 3-Brommethyl-phthalsaeure-anhydrid 40706-66-9 C9H5BrO3 241.041 —— 3-methyl-phthalic acid-1-methyl ester 14346-57-7 C10H10O4 194.187 二甲基3-甲基邻苯二甲酸酯 dimethyl 3-methylphthalate 21483-46-5 C11H12O4 208.214 —— 2-Carbomethoxy-3-methylbenzoesaeure 72288-71-2 C10H10O4 194.187 3-甲基邻苯二甲酸 3-methylphthalic acid 37102-74-2 C9H8O4 180.16 2-苯甲酰基-3-甲基苯甲酸甲酯 2-benzoyl-3-methyl-benzoic acid methyl ester 94501-26-5 C16H14O3 254.285 —— methyl 1,3-dihydro-3-oxo-4-isobenzofuranacetate 91345-00-5 C11H10O4 206.198 —— 3,3,7-trimethyl-3H-isobenzofuran-1-one 57732-90-8 C11H12O2 176.215 2-乙酰基-6-甲基-苯甲酸 6-Methyl-2-acetyl-benzoesaeure 56661-76-8 C10H10O3 178.188 —— 6-Methyl-2-benzoyl-benzoesaeure-methylester 20819-70-9 C16H14O3 254.285 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Normal and Pseudo Esters of 2-Benzoylbenzoic Acid Types1,2摘要:DOI:10.1021/ja01851a014
-
作为产物:描述:2,6-二甲基-N-(5-甲基-1,2-恶唑-3-基)苯甲酰胺 在 potassium permanganate 、 水 、 sodium carbonate 作用下, 以 水 为溶剂, 反应 4.0h, 生成 3-甲基邻苯二甲酸酐参考文献:名称:Chemical oxidation of an anticonvulsant N-(5′-methylisoxazol-3-yl)-2,6-dimethylbenzamide (D2916)摘要:The new anticonvulsant N-(5'-methylisoxazol-3-yl)-2,6-dimethylbenzamide (D2916), which presents two kinds of methyl groups which could be oxidized, was submitted to various chemical oxidizing agents. Several sites and degrees of oxidation were observed. The main oxidized site was the arylmethyl group without cleavage of the isoxazole ring, leading via carboxylic acid and primary alcohol intermediates to phthalimide and lactame derivatives. In no case was the methyl group of the isoxazole moiety hydroxylated. (C) 1998 Elsevier Science S.A. All rights reserved.DOI:10.1016/s0014-827x(98)00057-3
文献信息
-
Unmasking Amides: Ruthenium-Catalyzed Protodecarbonylation of N-Substituted Phthalimide Derivatives作者:Yu-Chao Yuan、Raghu Kamaraj、Christian Bruneau、Thierry Labasque、Thierry Roisnel、Rafael Gramage-DoriaDOI:10.1021/acs.orglett.7b03278日期:2017.12.1The unprecedented transformation of a wide range of synthetically appealing phthalimides into amides in a single-step operation has been achieved in high yields and short reaction times using a ruthenium catalyst. Mechanistic studies revealed a unique, homogeneous pathway involving five-membered ring opening and CO2 release with water being the source of protons.
-
NOVEL IMMUNOMODULATOR AND ANTI-INFLAMMATORY COMPOUNDS申请人:MUTHUPPALANIAPPAN Meyyappan公开号:US20110275603A1公开(公告)日:2011-11-10The present invention provides dihydroorotate dehydrogenase inhibitors, methods of preparing them, pharmaceutical compositions containing them and methods of treatment, prevention and/or amelioration of diseases or disorders wherein the inhibition of Dihydroorotate dehydrogenase is known to show beneficial effect.
-
调节WNT信号通路的酰胺类化合物及其用途申请人:中国医学科学院药物研究所公开号:CN109867661B公开(公告)日:2022-07-19本发明属于医药技术领域,特别涉及调节WNT信号通路的酰胺类化合物及其用途。根据本发明的化合物具有通式I所示的结构:
-
Characterization of Novel Aminobenzylcantharidinimides and Related Imides by Proton NMR Spectra and Their Effects on NO Induction作者:Ing-Jy Tseng、Pen-Yuan Lin、Shiow-Yunn Sheu、Wan-Ni Tung、Ching-Tung Lin、Mei-Hsiang LinDOI:10.1002/jccs.201400228日期:2015.10032) to produce cantharidinimides and their analogues in good yields. The para‐aminobenzylic imides showed greater inhibition of nitric oxide (NO) synthesis by NO synthase (NOS) than did ortho‐ and meta‐aminobenzylic imides. Compound 3fp, para‐aminobenzylic norbonane‐imide, had the most potent effect on inducible NOS among the tested compounds and showed 35% inhibition.通过与氨基苄胺和三乙胺反应,将包括邻th啶在内的各种酸酐转变为相应的氨基苄基邻ari啶亚胺3a和类似的酰亚胺3b〜k(在邻位,间位和对位)。邻苯二甲酰亚胺3ao,3am和3ap的两个甲基侧链以及相关的酰亚胺具有两个以上的手性中心。当苄胺的氨基在邻位时,氢的孤电子对在H-NMR光谱中显示出不同的化学位移和耦合常数位置。这些邻苯二甲酰亚胺具有母体苯胺,吡啶和萘的平面结构,伯胺的亲核性和碱性可能反映了感应电子对化学位移的负面影响。我们通过将反应物Cantharidin 1a,脂族和芳族酸酐,伯苄基胺和苯胺衍生物加热到大约17.5 ℃,制备了cantharidinimides 。在高压密封管(Buchi glasuster 0032)中,用3 mL干甲苯和1-2 mL三乙胺在200°C的条件下以高收率生产邻苯二甲酰亚胺和类似物。的对位-aminobenzylic酰亚胺表明一氧化氮(NO)合成的更大
-
Structure-Based Design, Parallel Synthesis, Structure−Activity Relationship, and Molecular Modeling Studies of Thiocarbamates, New Potent Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitor Isosteres of Phenethylthiazolylthiourea Derivatives作者:Angelo Ranise、Andrea Spallarossa、Sara Cesarini、Francesco Bondavalli、Silvia Schenone、Olga Bruno、Giulia Menozzi、Paola Fossa、Luisa Mosti、Massimiliano La Colla、Giuseppina Sanna、Marta Murreddu、Gabriella Collu、Bernardetta Busonera、Maria Elena Marongiu、Alessandra Pani、Paolo La Colla、Roberta LoddoDOI:10.1021/jm049252r日期:2005.6.1In this paper we describe our structure-based ligand design, synthetic strategy, and structure-activity relationship (SAR) studies that led to the identification of thiocarbamates (TCs), a novel class of non-nucleoside reverse transcriptase inhibitors (NNRTIs), isosteres of phenethylthiazolylthiourea (PETT) derivatives. Assuming as a lead compound O-[2-(phthalimido)ethyl]phenylthiocarbamate 12, one在本文中,我们描述了我们基于结构的配体设计,合成策略和结构与活性关系(SAR)的研究,这些研究导致了硫代氨基甲酸酯(TC)的鉴定,这是一类新型的非核苷类逆转录酶抑制剂(NNRTIs),等位基因苯乙基噻唑基硫脲(PETT)衍生物的制备。假设O- [2-(邻苯二甲酰亚胺基)乙基]苯基硫代氨基甲酸酯为先导化合物,前述酰基硫代氨基甲酸酯的前体之一(Ranise,A .;等人,J。Med。Chem。2003,46,768-781) ,通过平行合成制备了两个目标溶液相TC库。领先的优化策略导致对位取代的TC 31、33、34、39、40、41、44、45和50在纳摩尔浓度的基于MT-4的测定中对野生型HIV-1具有活性( EC50范围:0.04-0.01 microM)。最有效的同类物50(EC50 = 0。01 microM)在邻苯二甲酰亚胺部分的第4位带有一个甲基,在N-苯环的对位带有一个硝基。大
表征谱图
-
氢谱1HNMR
-
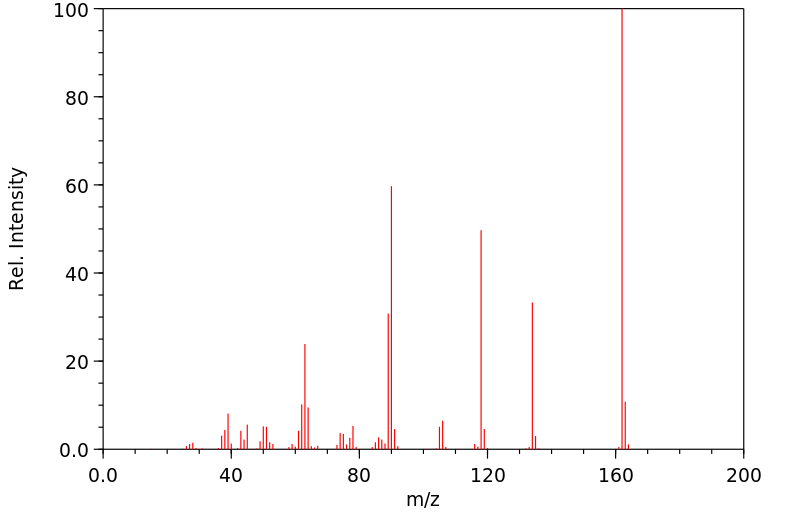
质谱MS
-
碳谱13CNMR
-
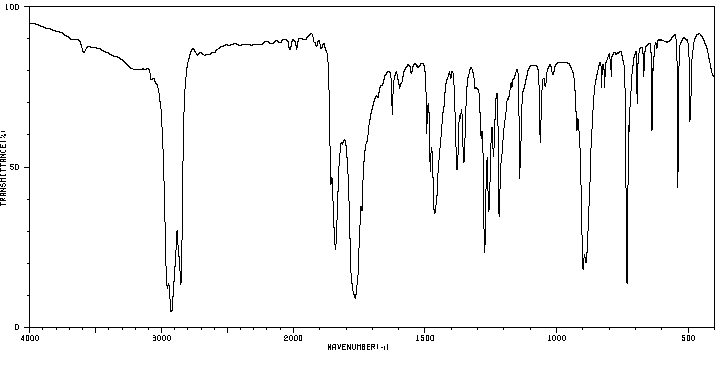
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
()-2-(5-甲基-2-氧代苯并呋喃-3(2)-亚乙基)乙酸乙酯
顺式-1-((2-(5-氯-2-苯并呋喃基)-4-甲基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
顺式-1-((2-(5,7-二氯-2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-咪唑
顺式-1-((2-(2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
霉酚酸酯杂质B
雷美替胺杂质3
雷美替胺杂质22
雷美替胺杂质
间甲酚紫
间甲基苯基(苯并呋喃-2-基)甲醇
长管假茉莉素C
钠1,4-二[(2-乙基己基)氧基]-1,4-二氧代-2-丁烷磺酸酯-3,3-二(4-羟基苯基)-2-苯并呋喃-1(3H)-酮(1:1:1)
金霉素
酪氨酸,b-羰基-
酞酸酐-d4
酚酞二丁酸酯
酚酞
酚红钠
酚红
邻苯二甲酸酐与马来酸酐,甘氨酰蜡素和二乙二醇的聚合物
邻苯二甲酸酐与己二醇的聚合物
邻苯二甲酸酐与三甘醇异壬醇的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇和2,5-呋喃二酮的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇、2,5-呋喃二酮和2-乙基己酸苯甲酸酯的聚合物
邻苯二甲酸酐-13C6
邻苯二甲酸酐-4-硼酸频哪醇酯
邻苯二甲酸酐,马来酸,二乙二醇,新戊二醇聚合物
邻甲酚酞二庚酸酯
邻甲酚酞二己酸酯
邻甲酚酞
贝康唑
表灰黄霉素
螺佐呋酮
螺[苯并呋喃-3(2H),4-哌啶]
螺[异苯并呋喃-1(3H),4’-哌啶]-3-酮
螺[异苯并呋喃-1(3H),4'-哌啶]-3-酮盐酸盐
螺[异苯并呋喃-1(3H),3’-吡咯烷]-3-酮
螺[1-苯并呋喃-2,1'-环丙烷]-3-酮
薄荷内酯
萘并[2,3-b]呋喃-8(4H)-酮,4a,5,6,7,8a,9-六氢-,顺-
莫罗卡尼
荨麻叶泽兰酮
荧光胺
苯酞-3-乙酸
苯酚,2-[3-(2-苯并呋喃基)-5,6-二氢-1,2,4-三唑并[3,4-b][1,3,4]噻二唑-6-基]-
苯酐二乙二醇共聚物
苯酐
苯甲酸,2-[(1,3-二羰基丁基)氨基]-,甲基酯
苯甲酸,2,2-二(羟甲基)丙烷-1,3-二醇,异苯并呋喃-1,3-二酮
苯甲酰氯化,3-甲氧基-4-甲基-