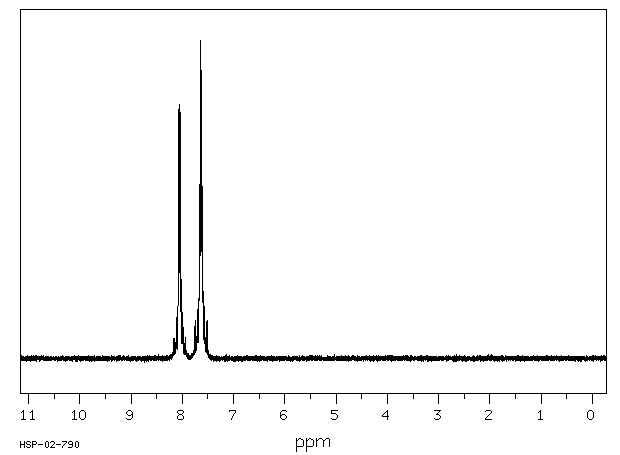
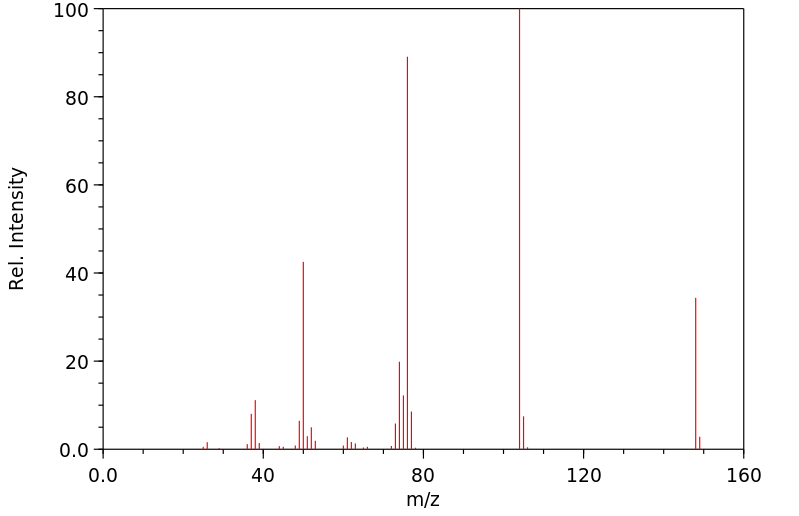
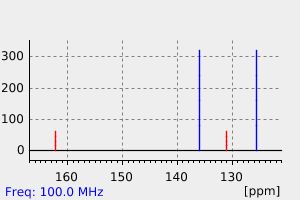
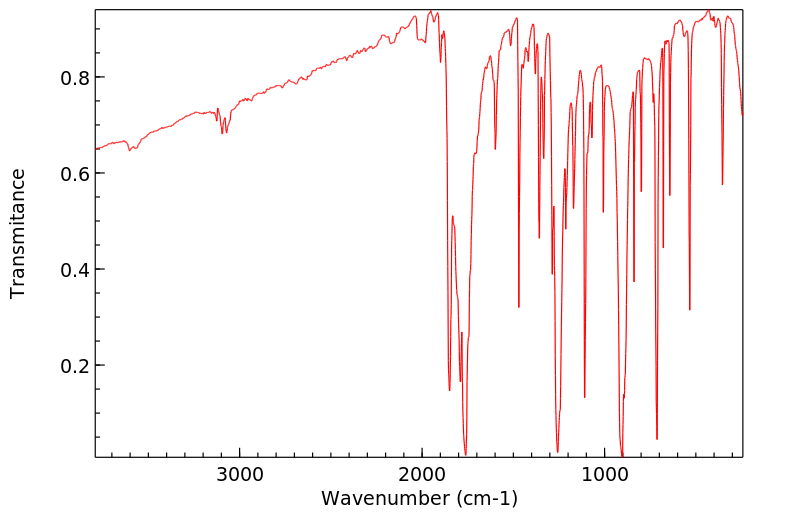
代谢
在一项研究中,调查了人类对苯酐的排泄情况,该研究收集了9名主要通过吸入途径职业暴露于苯酐的工人的尿液样本。样本在班前(7:00小时)、班中、班后(15:00小时)以及工作日当晚和次日早晨收集。工人呼吸区域的个人空气样本中测得的空气中苯酐水平从0.03至10.5毫克/立方米(平均值,MMAD未说明)。还收集了22名未职业暴露于苯酐的人的尿液作为对照组。在水的作用下,苯酐转化为苯二甲酸。尿液中的苯二甲酸浓度通过甲醇酯化后的电子捕获气相色谱法测量,并以尿肌酐表示。尿液样本还经历了酸、碱和酶水解,通过β-葡萄糖醛酸酶或芳基硫酸酯酶。在低大气苯酐浓度下(平均±标准差;0.15±0.15毫克/立方米,范围0.03-0.33毫克/立方米,n=5),苯二甲酸的排泄量从班前(7:00小时)浓度增加到班后(15:00小时)浓度,然后下降,直至再次达到班前浓度。班前尿液中苯二甲酸的浓度(0.49±0.15微摩尔/毫摩尔肌酐)与未职业暴露人群的浓度(0.34±0.25微摩尔/毫摩尔肌酐,范围0.02-0.089微摩尔/毫摩尔肌酐,n=22)没有显著差异。暴露于更高浓度的大气苯酐(1.63±0.13毫克/立方米,n=2)导致苯二甲酸的体内负荷在夜间未能完全清除,班前尿液中苯二甲酸的浓度平均是对照组的三倍(1.02±0.25微摩尔/毫摩尔肌酐)。一名暴露于高浓度苯酐(10.2毫克/立方米)的工人的班前尿液中苯二甲酸浓度为4.8微摩尔/毫摩尔肌酐;大约是对照组的14倍。尿液中苯二甲酸的浓度从班前水平增加到班后或晚上尿液样本中的最大值。然后浓度下降,半衰期约为14小时(关于半衰期估计没有更多信息)。没有观察到结合物的形成。因此,职业暴露于大气苯酐的工人吸收了该物质,其中一部分以非结合苯二甲酸的形式通过尿液排出。
The excretion of phthalic anhydride in humans has been investigated in a study where urine samples were collected from nine subjects occupationally exposed to phthalic anhydride, primarily by the inhalation route. Samples were taken pre-shift (7:00 hours) on-shift, post-shift (15:00 hours) and in the evening and the next morning after work day. Airborne phthalic anhydride levels ranged from 0.03 to 10.5 mg/cu m (mean value, MMAD not stated), determined from personal air samples from the worker breathing zone. Urine was also taken from a control group of 22 persons not occupational exposed to phthalic anhydride. Phthalic anhydride is converted to phthalic acid in the presence of water. Phthalic acid concentration in the urine was measured after esterification with methanol by electron capture gas chromatography, and expressed in terms of urinary creatinine. Urine samples were also subjected to acid, alkaline, and enzymatic hydrolysis by beta-glucuronidase or aryl sulphatase. At low atmospheric phthalic anhydride concentrations (mean +/- SD; 0.15 +/- 0.15 mg/cu m, range 0.03 - 0.33 mg/cu m, n=5) the excretion of phthalic acid increased from the pre-shift (7:00 hours) concentration to the post-shift (15:00 hours) concentration and decreased then until the pre-shift concentration was again reached. The pre-shift phthalic acid concentration in the urine (0.49 +/- 0.15 umol/mmol creatinine) were not significantly different from those of occupationally unexposed people (0.34 +/- 0.25 mol/mmol creatinine, range 0.02-0.089 umol/mmol creatinine, n=22). Exposure to higher concentrations of phthalic anhydride in air (1.63 +/- 0.13 mg/cu m, n=2) resulted in a body load of phthalic acid which was not totally cleared overnight, and with pre-shift phthalic acid concentrations in the urine with a mean value three times the mean control value (1.02 +/- 0.25 umol/mmol creatinine). One worker exposed to high concentration of phthalic anhydride (10.2 mg/cu m) had a pre-shift urinary concentration of 4.8 umol of phthalic acid /mmol creatinine; approximately 14 times that of the control group. The concentration of phthalic acid in the urine was found to increase from the pre-shift level to a maximum in the immediate postshift or evening urine sample. The concentration then decreased, with a half-life of approx. 14 hours (no further information on half-life estimation). No evidence was seen of conjugate formation. Thus, workers occupationally exposed to atmospheric phthalic anhydride absorbed the substance with some being excreted in the urine as unconjugated phthalic acid.
来源:Hazardous Substances Data Bank (HSDB)